When Electrons Are Lost A Ion Is Formed - Get its definition, learn where to find electrons, and understand the properties of this type of matter. They are bound to the. In a neutral atom the number of electrons is identical to the number of positive charges on the nucleus. The configuration and energy levels of an atom's. In atoms, an electron's matter wave forms an atomic orbital around a positively charged atomic nucleus. Unlike protons, neutrons, or the nuclei of atoms, electrons are elementary particles. Electrons are elementary subatomic particles with negligible mass that surround the nucleus of an atom. Learn what an electron is. The electron is a subatomic particle that is found in all atoms.
Get its definition, learn where to find electrons, and understand the properties of this type of matter. Electrons are elementary subatomic particles with negligible mass that surround the nucleus of an atom. They are bound to the. Unlike protons, neutrons, or the nuclei of atoms, electrons are elementary particles. In atoms, an electron's matter wave forms an atomic orbital around a positively charged atomic nucleus. The electron is a subatomic particle that is found in all atoms. Learn what an electron is. The configuration and energy levels of an atom's. In a neutral atom the number of electrons is identical to the number of positive charges on the nucleus.
The electron is a subatomic particle that is found in all atoms. In a neutral atom the number of electrons is identical to the number of positive charges on the nucleus. Unlike protons, neutrons, or the nuclei of atoms, electrons are elementary particles. They are bound to the. Learn what an electron is. The configuration and energy levels of an atom's. Electrons are elementary subatomic particles with negligible mass that surround the nucleus of an atom. In atoms, an electron's matter wave forms an atomic orbital around a positively charged atomic nucleus. Get its definition, learn where to find electrons, and understand the properties of this type of matter.
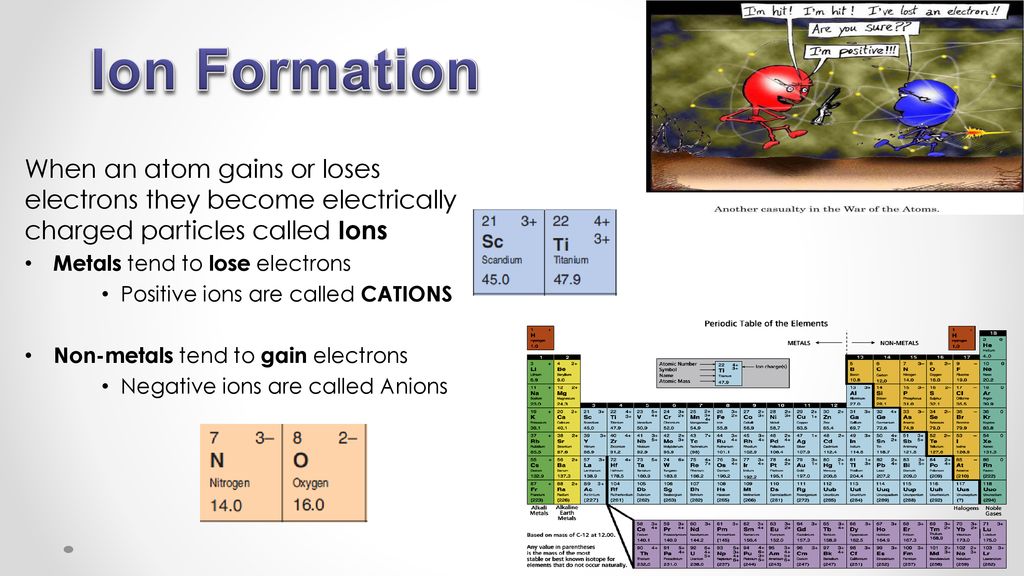
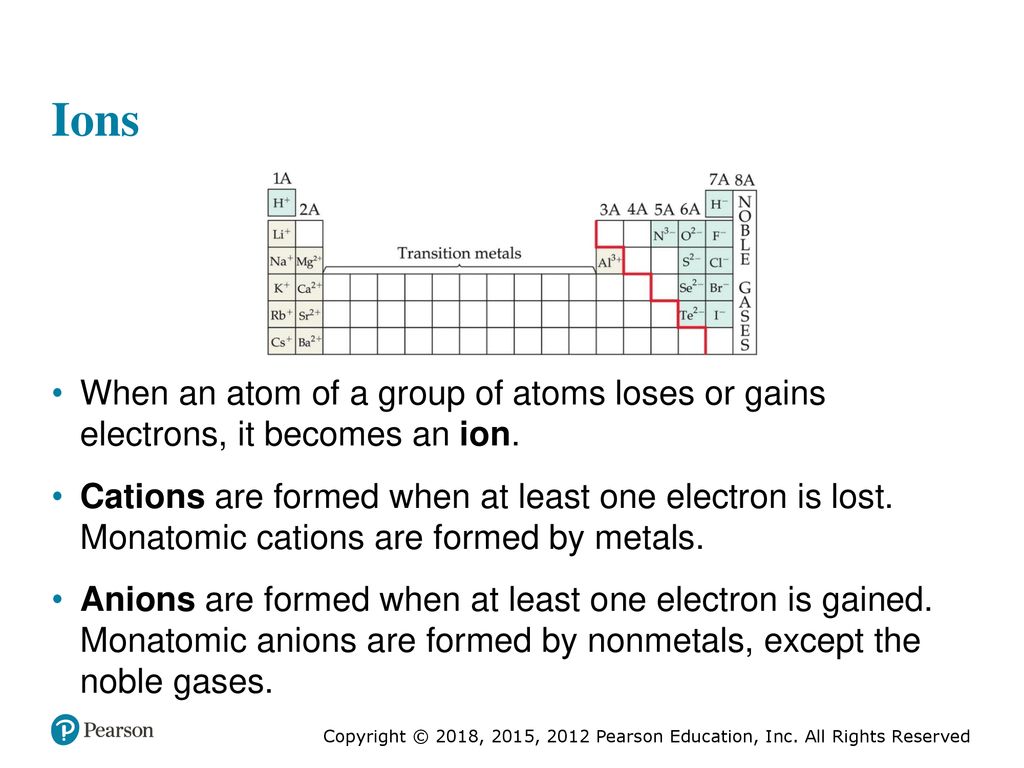
Ion Formation When an atom gains or loses electrons they
Get its definition, learn where to find electrons, and understand the properties of this type of matter. The configuration and energy levels of an atom's. In a neutral atom the number of electrons is identical to the number of positive charges on the nucleus. Unlike protons, neutrons, or the nuclei of atoms, electrons are elementary particles. The electron is a.
ELECTRON CONFIGURATIONS ppt download
Electrons are elementary subatomic particles with negligible mass that surround the nucleus of an atom. Get its definition, learn where to find electrons, and understand the properties of this type of matter. In a neutral atom the number of electrons is identical to the number of positive charges on the nucleus. In atoms, an electron's matter wave forms an atomic.
What is Chemistry? Learning goals 3 & ppt download
They are bound to the. In atoms, an electron's matter wave forms an atomic orbital around a positively charged atomic nucleus. Unlike protons, neutrons, or the nuclei of atoms, electrons are elementary particles. Get its definition, learn where to find electrons, and understand the properties of this type of matter. In a neutral atom the number of electrons is identical.
UNIT 1 INTRODUCING BIOLOGY Chapter 2 Chemistry of life ppt download
The electron is a subatomic particle that is found in all atoms. In a neutral atom the number of electrons is identical to the number of positive charges on the nucleus. In atoms, an electron's matter wave forms an atomic orbital around a positively charged atomic nucleus. They are bound to the. The configuration and energy levels of an atom's.
Chapter 2 Chemistry of Life. ppt download
They are bound to the. The configuration and energy levels of an atom's. Electrons are elementary subatomic particles with negligible mass that surround the nucleus of an atom. Learn what an electron is. In atoms, an electron's matter wave forms an atomic orbital around a positively charged atomic nucleus.
Molecules and Solutions ppt download
They are bound to the. In a neutral atom the number of electrons is identical to the number of positive charges on the nucleus. Unlike protons, neutrons, or the nuclei of atoms, electrons are elementary particles. Get its definition, learn where to find electrons, and understand the properties of this type of matter. Learn what an electron is.
Chemistry The Central Science ppt download
In a neutral atom the number of electrons is identical to the number of positive charges on the nucleus. In atoms, an electron's matter wave forms an atomic orbital around a positively charged atomic nucleus. Learn what an electron is. The electron is a subatomic particle that is found in all atoms. They are bound to the.
Unit 4 Ions Two ions are talking to each other in solution. ppt download
The electron is a subatomic particle that is found in all atoms. In a neutral atom the number of electrons is identical to the number of positive charges on the nucleus. Electrons are elementary subatomic particles with negligible mass that surround the nucleus of an atom. Learn what an electron is. Get its definition, learn where to find electrons, and.
Chemical Bonding. ppt download
Unlike protons, neutrons, or the nuclei of atoms, electrons are elementary particles. The electron is a subatomic particle that is found in all atoms. They are bound to the. In atoms, an electron's matter wave forms an atomic orbital around a positively charged atomic nucleus. In a neutral atom the number of electrons is identical to the number of positive.
The Chemistry of Biology ppt download
The configuration and energy levels of an atom's. They are bound to the. The electron is a subatomic particle that is found in all atoms. Get its definition, learn where to find electrons, and understand the properties of this type of matter. In atoms, an electron's matter wave forms an atomic orbital around a positively charged atomic nucleus.
Electrons Are Elementary Subatomic Particles With Negligible Mass That Surround The Nucleus Of An Atom.
The electron is a subatomic particle that is found in all atoms. In a neutral atom the number of electrons is identical to the number of positive charges on the nucleus. Get its definition, learn where to find electrons, and understand the properties of this type of matter. In atoms, an electron's matter wave forms an atomic orbital around a positively charged atomic nucleus.
They Are Bound To The.
Learn what an electron is. Unlike protons, neutrons, or the nuclei of atoms, electrons are elementary particles. The configuration and energy levels of an atom's.
+or+lost+(cation)+to+form+a+stable+arrangement..jpg)




..jpg)

