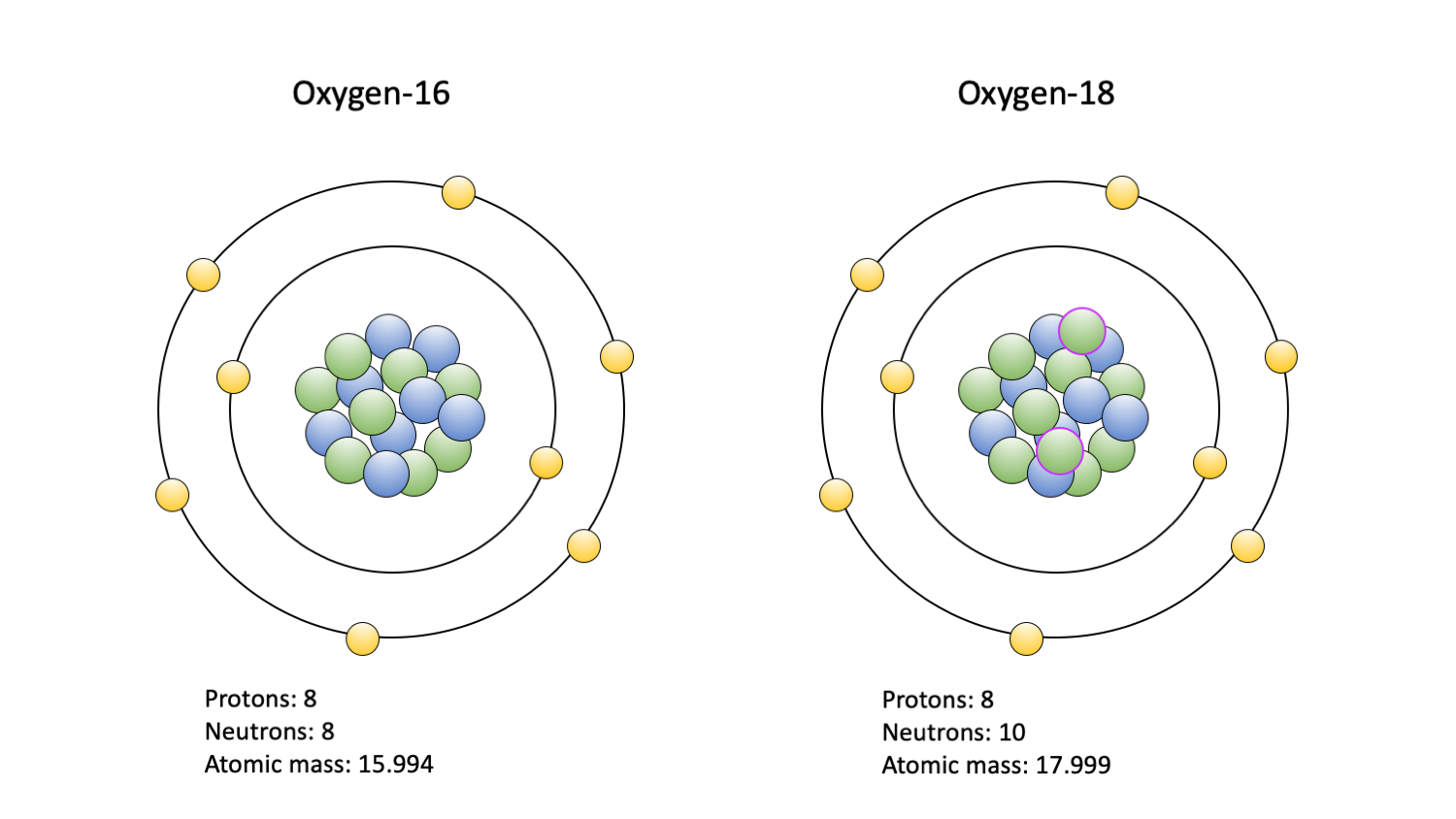
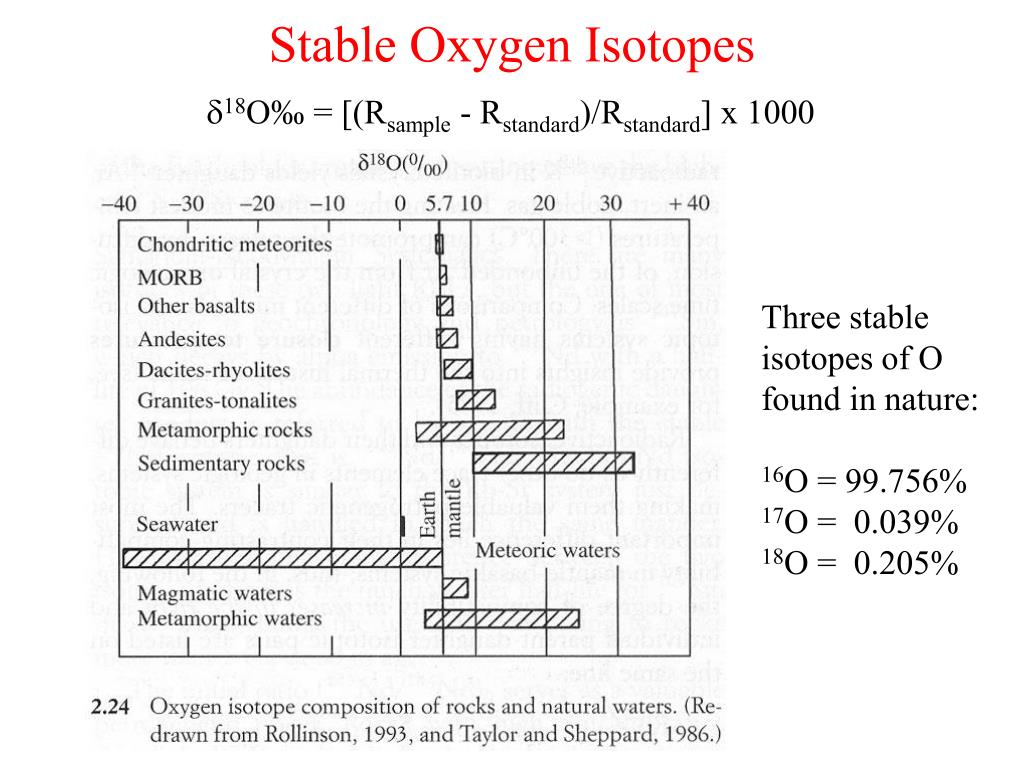
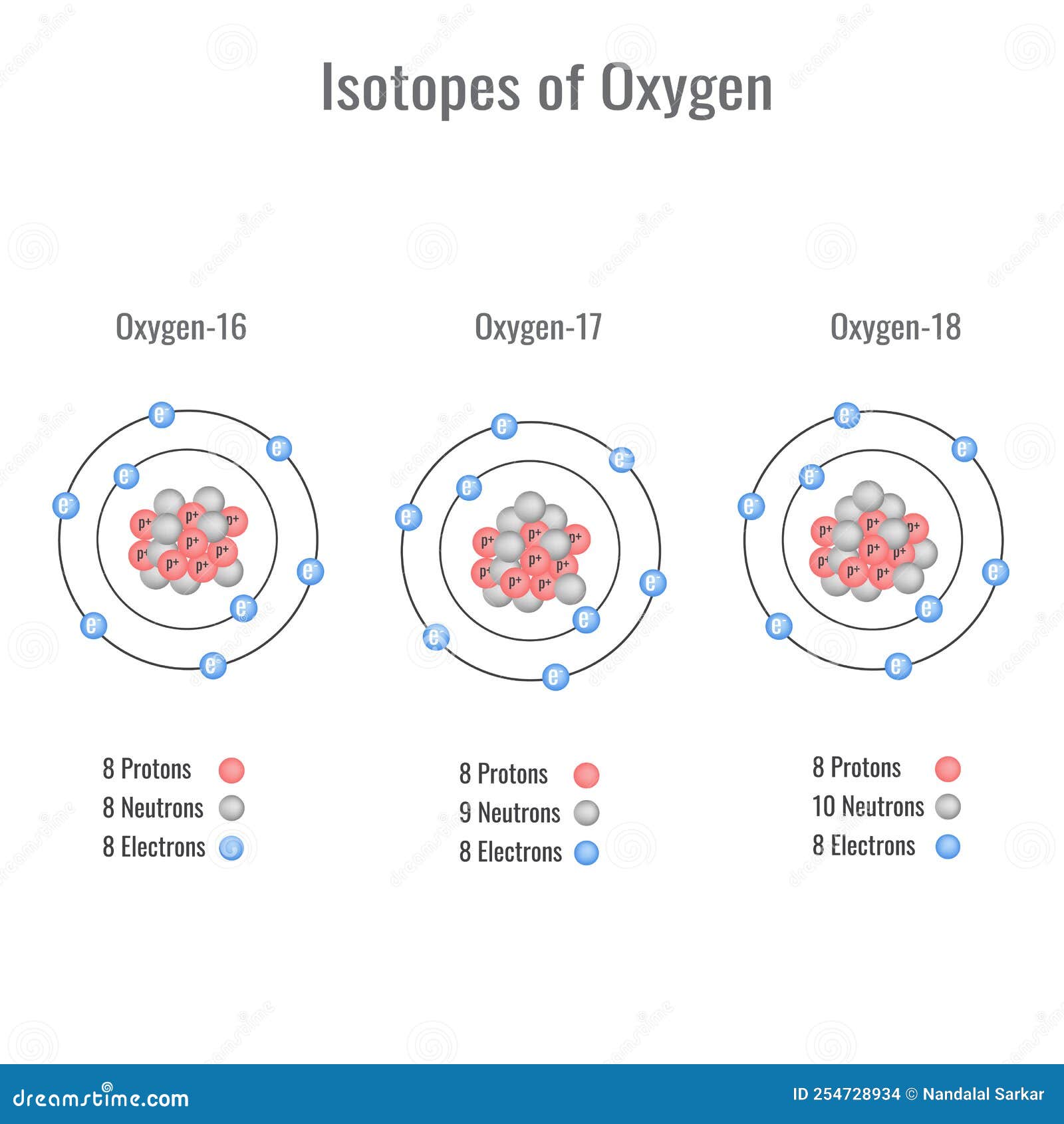
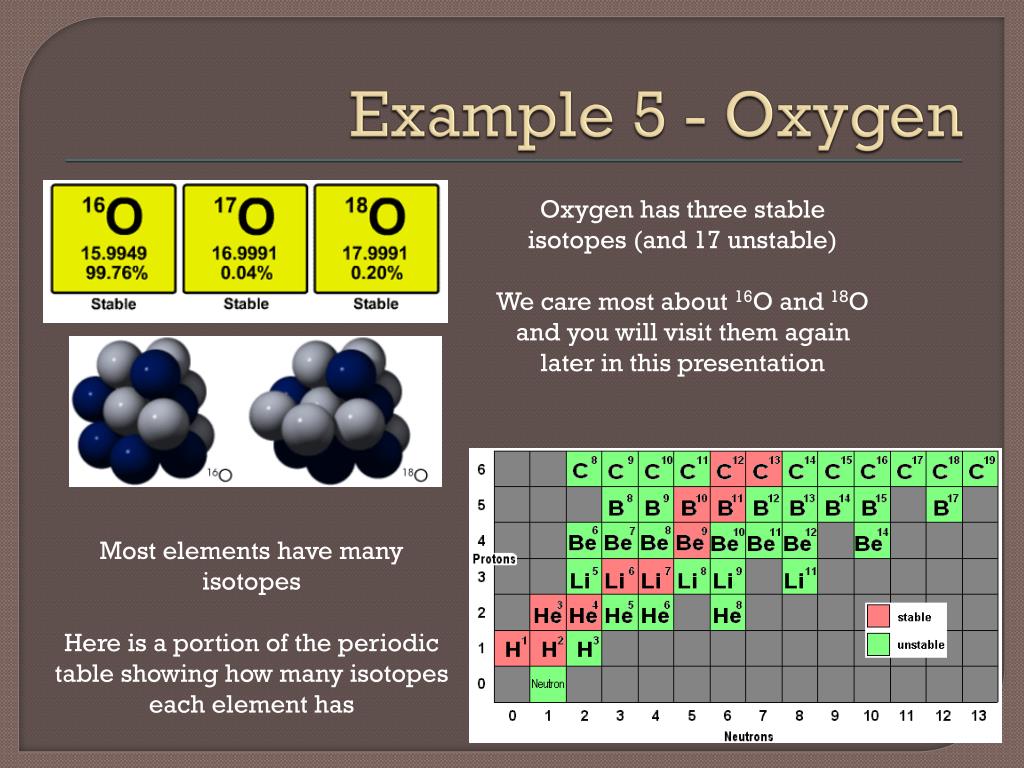
Stable Forms Of Oxygen - Oxygen is in group 16 of the periodic table, so it has 6 valence electrons and can gain 2 electrons to. Isotope oxygen is a variant of the element oxygen that has a different number of neutrons in its nucleus. No, oxygen typically forms 2 bonds. Oh, dude, you're talking about the stable forms of oxygen? Oxygen atoms need to share or gain two electrons in order to achieve a stable electron configuration. What is isotope oxygen is?
Oxygen is in group 16 of the periodic table, so it has 6 valence electrons and can gain 2 electrons to. What is isotope oxygen is? Isotope oxygen is a variant of the element oxygen that has a different number of neutrons in its nucleus. No, oxygen typically forms 2 bonds. Oxygen atoms need to share or gain two electrons in order to achieve a stable electron configuration. Oh, dude, you're talking about the stable forms of oxygen?
No, oxygen typically forms 2 bonds. Oxygen is in group 16 of the periodic table, so it has 6 valence electrons and can gain 2 electrons to. What is isotope oxygen is? Isotope oxygen is a variant of the element oxygen that has a different number of neutrons in its nucleus. Oh, dude, you're talking about the stable forms of oxygen? Oxygen atoms need to share or gain two electrons in order to achieve a stable electron configuration.
Thermochemistry Thermodynamics Gases Paul Franklyn C305 Consultation
Isotope oxygen is a variant of the element oxygen that has a different number of neutrons in its nucleus. What is isotope oxygen is? Oxygen is in group 16 of the periodic table, so it has 6 valence electrons and can gain 2 electrons to. Oxygen atoms need to share or gain two electrons in order to achieve a stable.
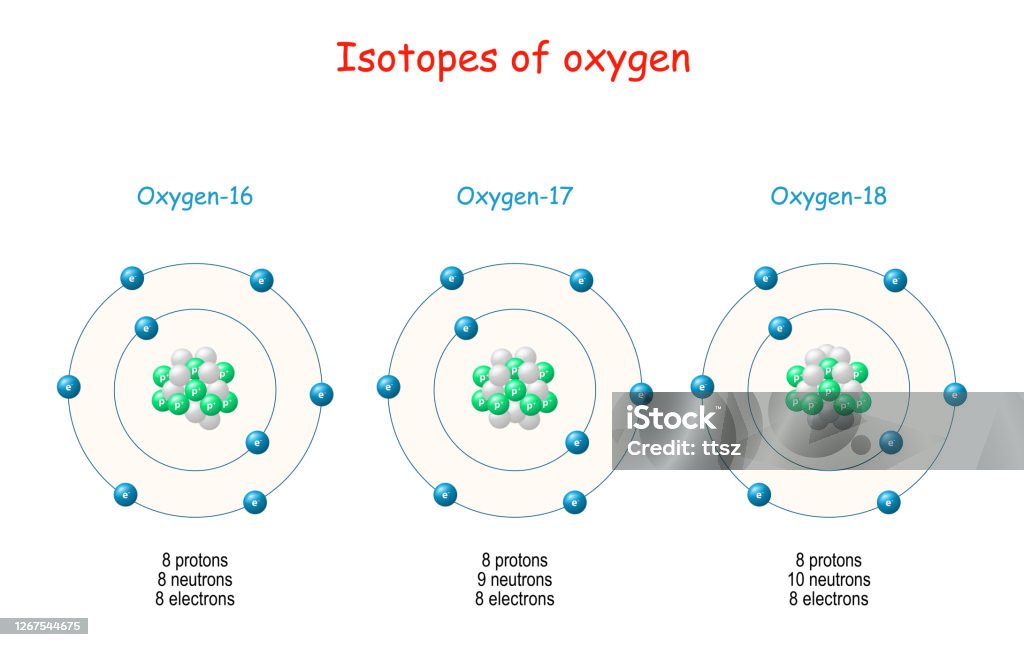
Isotopes de l'oxygène illustration de vecteur. Illustration du chemical
Oxygen atoms need to share or gain two electrons in order to achieve a stable electron configuration. Oh, dude, you're talking about the stable forms of oxygen? Isotope oxygen is a variant of the element oxygen that has a different number of neutrons in its nucleus. What is isotope oxygen is? No, oxygen typically forms 2 bonds.
Ionic Bonding. ppt download
Oxygen atoms need to share or gain two electrons in order to achieve a stable electron configuration. What is isotope oxygen is? Oh, dude, you're talking about the stable forms of oxygen? No, oxygen typically forms 2 bonds. Oxygen is in group 16 of the periodic table, so it has 6 valence electrons and can gain 2 electrons to.
Biogeochemical analysis and Paleoecology Digital Atlas of Ancient Life
Oxygen atoms need to share or gain two electrons in order to achieve a stable electron configuration. Oxygen is in group 16 of the periodic table, so it has 6 valence electrons and can gain 2 electrons to. What is isotope oxygen is? Isotope oxygen is a variant of the element oxygen that has a different number of neutrons in.
PPT Trace Elements Definitions PowerPoint Presentation, free
Isotope oxygen is a variant of the element oxygen that has a different number of neutrons in its nucleus. What is isotope oxygen is? Oxygen is in group 16 of the periodic table, so it has 6 valence electrons and can gain 2 electrons to. Oh, dude, you're talking about the stable forms of oxygen? Oxygen atoms need to share.
Element ' X ' forms five stable oxides with oxygen of formula X2 O,XO2 ,X..
What is isotope oxygen is? Oxygen atoms need to share or gain two electrons in order to achieve a stable electron configuration. Oxygen is in group 16 of the periodic table, so it has 6 valence electrons and can gain 2 electrons to. Oh, dude, you're talking about the stable forms of oxygen? No, oxygen typically forms 2 bonds.
Isotope Symbol
Oh, dude, you're talking about the stable forms of oxygen? Oxygen is in group 16 of the periodic table, so it has 6 valence electrons and can gain 2 electrons to. No, oxygen typically forms 2 bonds. Oxygen atoms need to share or gain two electrons in order to achieve a stable electron configuration. Isotope oxygen is a variant of.
PPT Isotopes, Ice Cores and Climate Change PowerPoint Presentation
Isotope oxygen is a variant of the element oxygen that has a different number of neutrons in its nucleus. What is isotope oxygen is? No, oxygen typically forms 2 bonds. Oxygen atoms need to share or gain two electrons in order to achieve a stable electron configuration. Oh, dude, you're talking about the stable forms of oxygen?
Stable Atom Over 400 RoyaltyFree Licensable Stock Vectors & Vector
What is isotope oxygen is? Oxygen is in group 16 of the periodic table, so it has 6 valence electrons and can gain 2 electrons to. No, oxygen typically forms 2 bonds. Isotope oxygen is a variant of the element oxygen that has a different number of neutrons in its nucleus. Oh, dude, you're talking about the stable forms of.
Isotopes Of Oxygen Stock Illustration Download Image Now Isotope
No, oxygen typically forms 2 bonds. Oxygen atoms need to share or gain two electrons in order to achieve a stable electron configuration. Oh, dude, you're talking about the stable forms of oxygen? Oxygen is in group 16 of the periodic table, so it has 6 valence electrons and can gain 2 electrons to. What is isotope oxygen is?
Oxygen Atoms Need To Share Or Gain Two Electrons In Order To Achieve A Stable Electron Configuration.
No, oxygen typically forms 2 bonds. Isotope oxygen is a variant of the element oxygen that has a different number of neutrons in its nucleus. What is isotope oxygen is? Oxygen is in group 16 of the periodic table, so it has 6 valence electrons and can gain 2 electrons to.