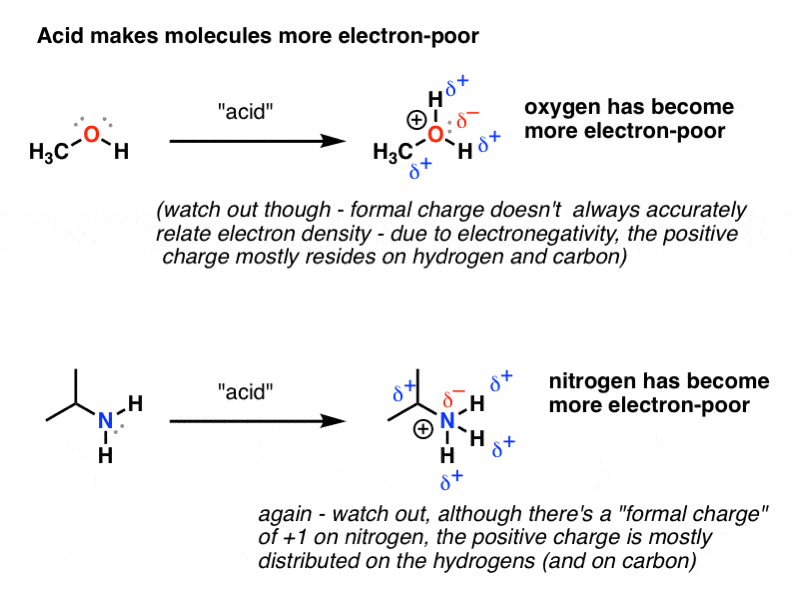
Protonated Form - Determine, based on the p k a values, if each of the following compounds can be protonated by water. Write the corresponding chemical equation and. In the example of the alcohol and the amine below, for instance, addition of acid. The opposite applies for molecules that are protonated. Protonation is usually reversible, and the structure and bonding of the conjugate base are normally unchanged on protonation. That is, if you find the compound at a particular ph, how many of the protons are on the molecule (in the protonated form) and how many are off the. When a species is either protonated or depronated, its mass and charge change, plus its chemical properties are altered.
Determine, based on the p k a values, if each of the following compounds can be protonated by water. When a species is either protonated or depronated, its mass and charge change, plus its chemical properties are altered. Write the corresponding chemical equation and. In the example of the alcohol and the amine below, for instance, addition of acid. The opposite applies for molecules that are protonated. Protonation is usually reversible, and the structure and bonding of the conjugate base are normally unchanged on protonation. That is, if you find the compound at a particular ph, how many of the protons are on the molecule (in the protonated form) and how many are off the.
The opposite applies for molecules that are protonated. When a species is either protonated or depronated, its mass and charge change, plus its chemical properties are altered. Write the corresponding chemical equation and. In the example of the alcohol and the amine below, for instance, addition of acid. Determine, based on the p k a values, if each of the following compounds can be protonated by water. That is, if you find the compound at a particular ph, how many of the protons are on the molecule (in the protonated form) and how many are off the. Protonation is usually reversible, and the structure and bonding of the conjugate base are normally unchanged on protonation.
The structures of the protonated forms of the Pbn clusters optimized at
In the example of the alcohol and the amine below, for instance, addition of acid. Write the corresponding chemical equation and. Protonation is usually reversible, and the structure and bonding of the conjugate base are normally unchanged on protonation. The opposite applies for molecules that are protonated. Determine, based on the p k a values, if each of the following.
Ground state structures of various protonation forms of the SAL3
When a species is either protonated or depronated, its mass and charge change, plus its chemical properties are altered. Protonation is usually reversible, and the structure and bonding of the conjugate base are normally unchanged on protonation. Write the corresponding chemical equation and. That is, if you find the compound at a particular ph, how many of the protons are.
012Polyprotic Acids; Protonation State YouTube
Write the corresponding chemical equation and. Protonation is usually reversible, and the structure and bonding of the conjugate base are normally unchanged on protonation. That is, if you find the compound at a particular ph, how many of the protons are on the molecule (in the protonated form) and how many are off the. In the example of the alcohol.
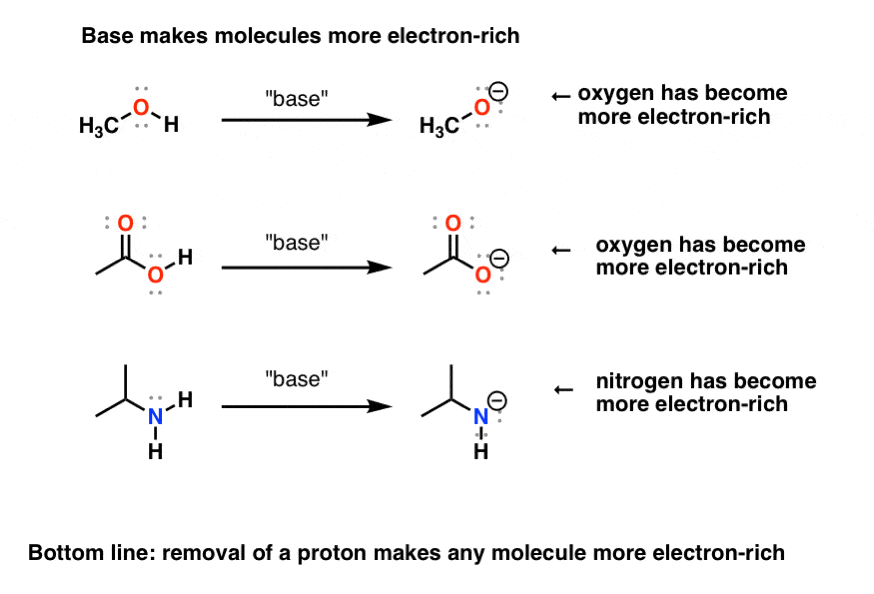
Protonation And Deprotonation Reactions Dramatic Effects On Reactivity
That is, if you find the compound at a particular ph, how many of the protons are on the molecule (in the protonated form) and how many are off the. When a species is either protonated or depronated, its mass and charge change, plus its chemical properties are altered. Determine, based on the p k a values, if each of.
Protonation And Deprotonation Reactions Dramatic Effects On Reactivity
The opposite applies for molecules that are protonated. In the example of the alcohol and the amine below, for instance, addition of acid. Write the corresponding chemical equation and. Determine, based on the p k a values, if each of the following compounds can be protonated by water. That is, if you find the compound at a particular ph, how.
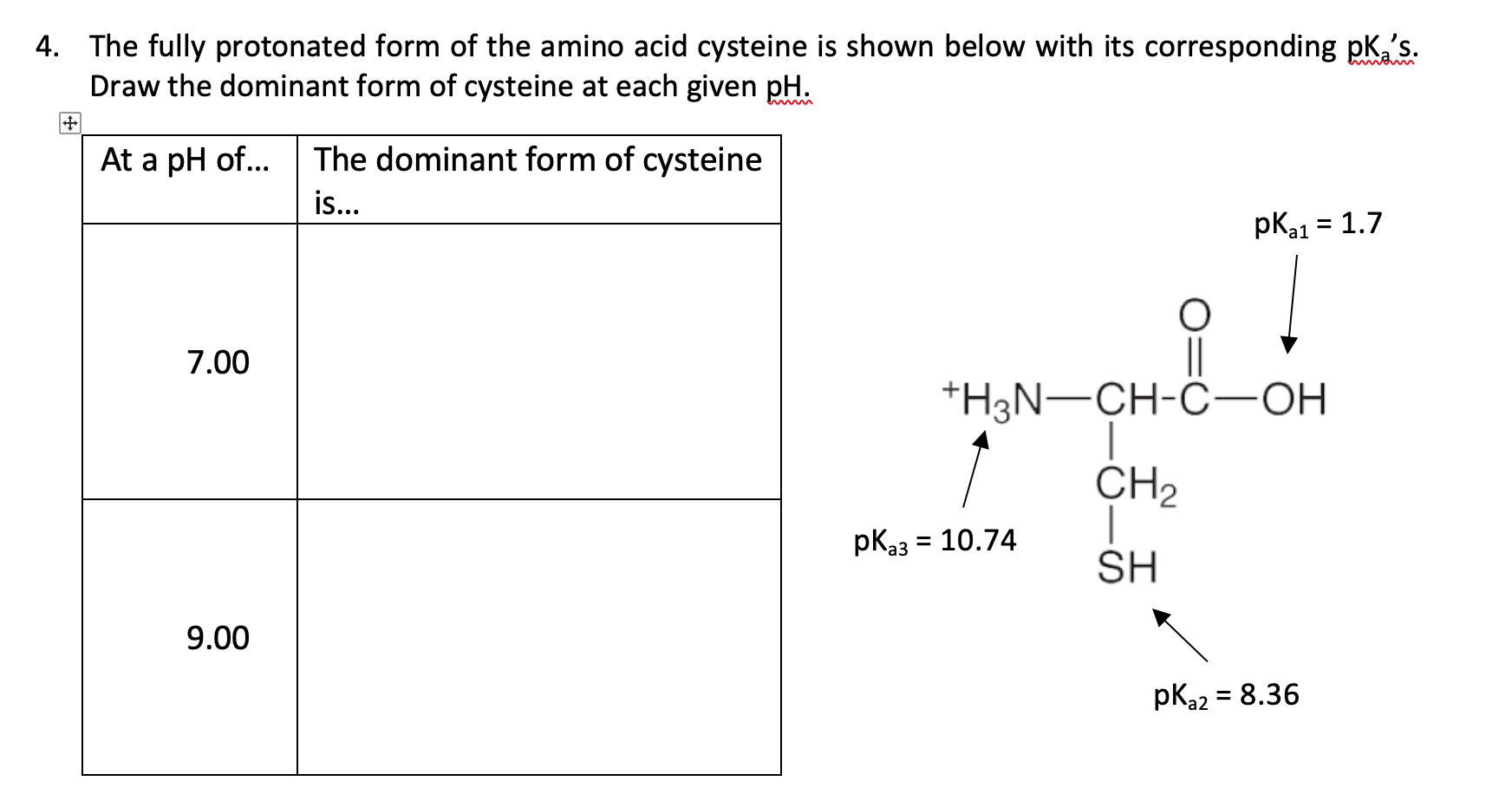
Solved 4. The fully protonated form of the amino acid
That is, if you find the compound at a particular ph, how many of the protons are on the molecule (in the protonated form) and how many are off the. Determine, based on the p k a values, if each of the following compounds can be protonated by water. Protonation is usually reversible, and the structure and bonding of the.
(a) The equilibrium between protonated form and deprotonated form of
That is, if you find the compound at a particular ph, how many of the protons are on the molecule (in the protonated form) and how many are off the. The opposite applies for molecules that are protonated. Write the corresponding chemical equation and. In the example of the alcohol and the amine below, for instance, addition of acid. When.
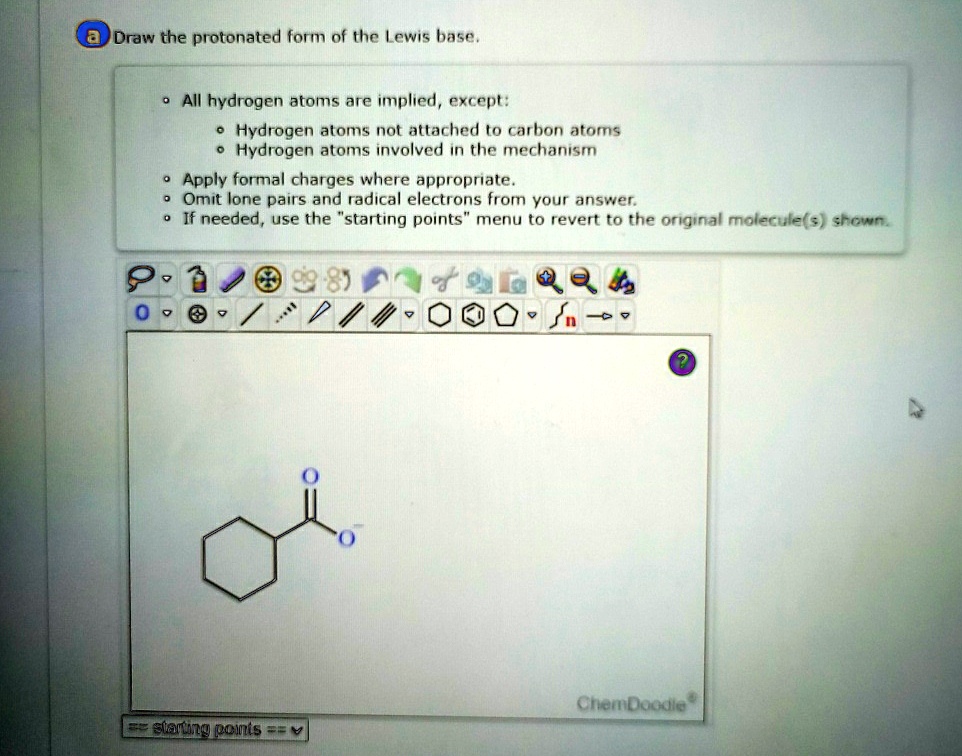
SOLVED (a) Draw the protonated form of the Lewis base. All hydrogen
In the example of the alcohol and the amine below, for instance, addition of acid. Protonation is usually reversible, and the structure and bonding of the conjugate base are normally unchanged on protonation. That is, if you find the compound at a particular ph, how many of the protons are on the molecule (in the protonated form) and how many.
Protonation And Deprotonation Reactions Dramatic Effects On Reactivity
Determine, based on the p k a values, if each of the following compounds can be protonated by water. When a species is either protonated or depronated, its mass and charge change, plus its chemical properties are altered. Protonation is usually reversible, and the structure and bonding of the conjugate base are normally unchanged on protonation. That is, if you.
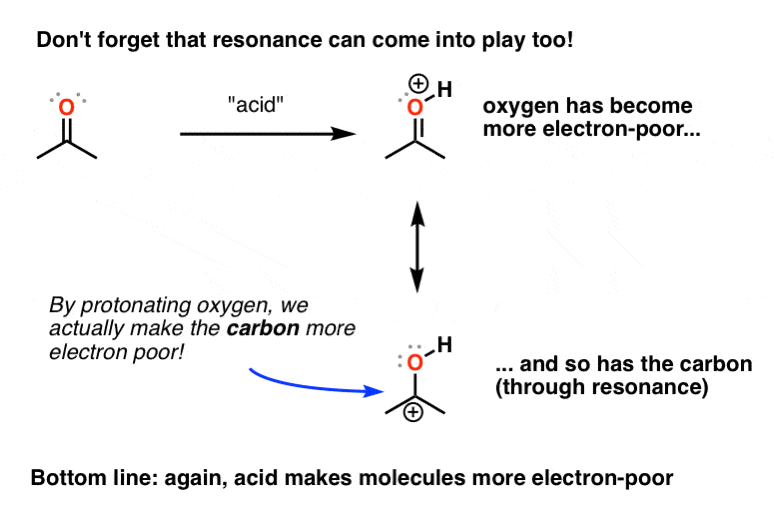
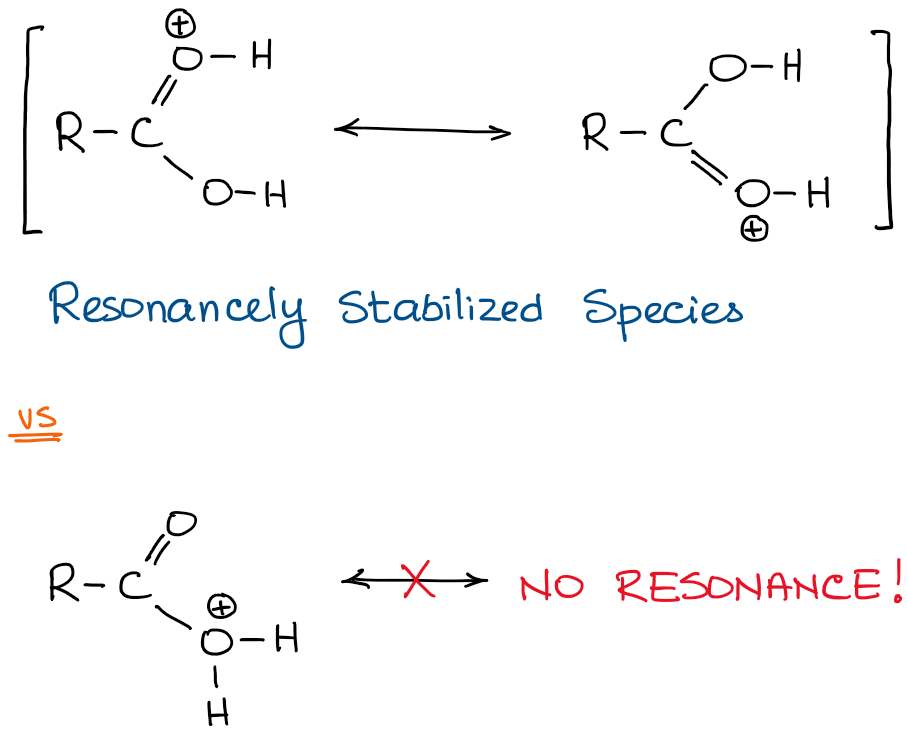
Protonating A Carboxylic Acid Which Atom To Choose? — Organic
The opposite applies for molecules that are protonated. When a species is either protonated or depronated, its mass and charge change, plus its chemical properties are altered. That is, if you find the compound at a particular ph, how many of the protons are on the molecule (in the protonated form) and how many are off the. Write the corresponding.
Determine, Based On The P K A Values, If Each Of The Following Compounds Can Be Protonated By Water.
When a species is either protonated or depronated, its mass and charge change, plus its chemical properties are altered. Write the corresponding chemical equation and. Protonation is usually reversible, and the structure and bonding of the conjugate base are normally unchanged on protonation. The opposite applies for molecules that are protonated.
That Is, If You Find The Compound At A Particular Ph, How Many Of The Protons Are On The Molecule (In The Protonated Form) And How Many Are Off The.
In the example of the alcohol and the amine below, for instance, addition of acid.