Pagibig Form - How do electrophilic aromatic substitution reactions on pyrrole, pyridine, naphthalene, or other groups compare? So how does halogenation of alkenes actually work? When it does undergo reaction with halogens, it occurs via. Electrophilic aromatic substitution (eas) reactions involve the substitution of hydrogen on an aromatic ring with an electrophile. Halogenation of benzene via electrophilic aromatic substitution. One initial idea was that they might proceed through a free carbocation intermediate,.
Electrophilic aromatic substitution (eas) reactions involve the substitution of hydrogen on an aromatic ring with an electrophile. When it does undergo reaction with halogens, it occurs via. Halogenation of benzene via electrophilic aromatic substitution. So how does halogenation of alkenes actually work? One initial idea was that they might proceed through a free carbocation intermediate,. How do electrophilic aromatic substitution reactions on pyrrole, pyridine, naphthalene, or other groups compare?
So how does halogenation of alkenes actually work? Halogenation of benzene via electrophilic aromatic substitution. How do electrophilic aromatic substitution reactions on pyrrole, pyridine, naphthalene, or other groups compare? One initial idea was that they might proceed through a free carbocation intermediate,. Electrophilic aromatic substitution (eas) reactions involve the substitution of hydrogen on an aromatic ring with an electrophile. When it does undergo reaction with halogens, it occurs via.
20112025 Form PH PagIBIG FPF040 Fill Online, Printable, Fillable
How do electrophilic aromatic substitution reactions on pyrrole, pyridine, naphthalene, or other groups compare? So how does halogenation of alkenes actually work? Halogenation of benzene via electrophilic aromatic substitution. One initial idea was that they might proceed through a free carbocation intermediate,. When it does undergo reaction with halogens, it occurs via.
Pagibig Calamity Loan form PagIBIG Fund P21a PagIBIG CALAMITY LOAN
Halogenation of benzene via electrophilic aromatic substitution. How do electrophilic aromatic substitution reactions on pyrrole, pyridine, naphthalene, or other groups compare? One initial idea was that they might proceed through a free carbocation intermediate,. When it does undergo reaction with halogens, it occurs via. Electrophilic aromatic substitution (eas) reactions involve the substitution of hydrogen on an aromatic ring with an.
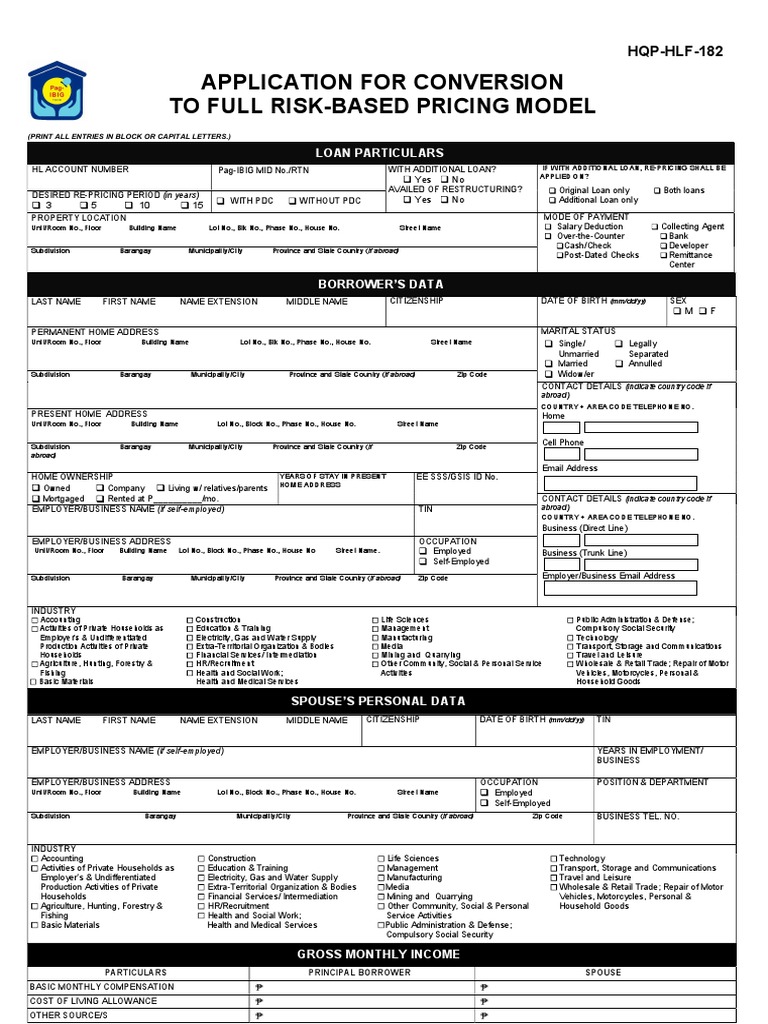
PAGIBIG FORM.pdf Credit (Finance) Loans
Electrophilic aromatic substitution (eas) reactions involve the substitution of hydrogen on an aromatic ring with an electrophile. Halogenation of benzene via electrophilic aromatic substitution. How do electrophilic aromatic substitution reactions on pyrrole, pyridine, naphthalene, or other groups compare? So how does halogenation of alkenes actually work? When it does undergo reaction with halogens, it occurs via.
Pag Ibig Members Data Form Update Victorialasopa Ceip vrogue.co
One initial idea was that they might proceed through a free carbocation intermediate,. Electrophilic aromatic substitution (eas) reactions involve the substitution of hydrogen on an aromatic ring with an electrophile. How do electrophilic aromatic substitution reactions on pyrrole, pyridine, naphthalene, or other groups compare? So how does halogenation of alkenes actually work? Halogenation of benzene via electrophilic aromatic substitution.
Virtual PagIBIG
How do electrophilic aromatic substitution reactions on pyrrole, pyridine, naphthalene, or other groups compare? One initial idea was that they might proceed through a free carbocation intermediate,. So how does halogenation of alkenes actually work? Electrophilic aromatic substitution (eas) reactions involve the substitution of hydrogen on an aromatic ring with an electrophile. When it does undergo reaction with halogens, it.
Employer'S Virtual PagIbig Enrollment Form PDF Signature Employment
So how does halogenation of alkenes actually work? How do electrophilic aromatic substitution reactions on pyrrole, pyridine, naphthalene, or other groups compare? Halogenation of benzene via electrophilic aromatic substitution. One initial idea was that they might proceed through a free carbocation intermediate,. When it does undergo reaction with halogens, it occurs via.
Pag Ibig Form ≡ Fill Out Printable PDF Forms Online
So how does halogenation of alkenes actually work? How do electrophilic aromatic substitution reactions on pyrrole, pyridine, naphthalene, or other groups compare? One initial idea was that they might proceed through a free carbocation intermediate,. Electrophilic aromatic substitution (eas) reactions involve the substitution of hydrogen on an aromatic ring with an electrophile. When it does undergo reaction with halogens, it.
Pag Ibig Loan Form 20202022 Fill and Sign Printable Template Online
When it does undergo reaction with halogens, it occurs via. One initial idea was that they might proceed through a free carbocation intermediate,. How do electrophilic aromatic substitution reactions on pyrrole, pyridine, naphthalene, or other groups compare? Electrophilic aromatic substitution (eas) reactions involve the substitution of hydrogen on an aromatic ring with an electrophile. So how does halogenation of alkenes.
PagIbig Fund LOYALTY CARD PLUS Application Form Online YouTube
When it does undergo reaction with halogens, it occurs via. Halogenation of benzene via electrophilic aromatic substitution. So how does halogenation of alkenes actually work? Electrophilic aromatic substitution (eas) reactions involve the substitution of hydrogen on an aromatic ring with an electrophile. One initial idea was that they might proceed through a free carbocation intermediate,.
Pag Ibig Form Mp2Rf ≡ Fill Out Printable PDF Forms Online
How do electrophilic aromatic substitution reactions on pyrrole, pyridine, naphthalene, or other groups compare? One initial idea was that they might proceed through a free carbocation intermediate,. Electrophilic aromatic substitution (eas) reactions involve the substitution of hydrogen on an aromatic ring with an electrophile. When it does undergo reaction with halogens, it occurs via. So how does halogenation of alkenes.
Electrophilic Aromatic Substitution (Eas) Reactions Involve The Substitution Of Hydrogen On An Aromatic Ring With An Electrophile.
How do electrophilic aromatic substitution reactions on pyrrole, pyridine, naphthalene, or other groups compare? Halogenation of benzene via electrophilic aromatic substitution. So how does halogenation of alkenes actually work? When it does undergo reaction with halogens, it occurs via.


.jpg)




