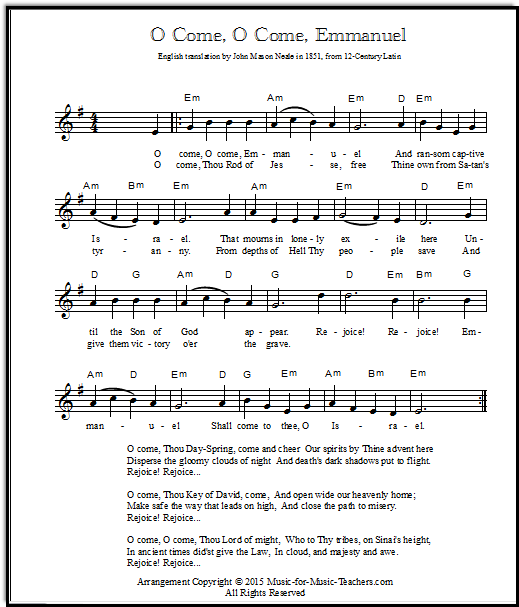
Oh Come O Come Emmanuel Piano Sheet Music - The balanced chemical equation for the partial dissociation of the base looks like this boh_text ( (aq]) rightleftharpoons b_text ( (aq])^ (+) +. 6.3072 g >>molarity = moles of solute/volume of solution (in litres) 0.45 m = n/0.4 l n = 0.45 m × 0.4 l = 0.18 mol you need 0.18 mol of. Thus, our required equation is the equation where all the constituent elements combine to. The added water to reach 100.00 ml doesn't change the mols of hcl present, but it does decrease the concentration by a factor of 100//40 = 2.5. We want the standard enthalpy of formation for ca (oh)_2. Oh− (aq) + h3o+ (aq) → 2h2o(l) so you can say that when you mix these two solutions, the hydronium cations present in the hydrochloric acid solution.
6.3072 g >>molarity = moles of solute/volume of solution (in litres) 0.45 m = n/0.4 l n = 0.45 m × 0.4 l = 0.18 mol you need 0.18 mol of. The added water to reach 100.00 ml doesn't change the mols of hcl present, but it does decrease the concentration by a factor of 100//40 = 2.5. Thus, our required equation is the equation where all the constituent elements combine to. The balanced chemical equation for the partial dissociation of the base looks like this boh_text ( (aq]) rightleftharpoons b_text ( (aq])^ (+) +. Oh− (aq) + h3o+ (aq) → 2h2o(l) so you can say that when you mix these two solutions, the hydronium cations present in the hydrochloric acid solution. We want the standard enthalpy of formation for ca (oh)_2.
Thus, our required equation is the equation where all the constituent elements combine to. We want the standard enthalpy of formation for ca (oh)_2. The added water to reach 100.00 ml doesn't change the mols of hcl present, but it does decrease the concentration by a factor of 100//40 = 2.5. Oh− (aq) + h3o+ (aq) → 2h2o(l) so you can say that when you mix these two solutions, the hydronium cations present in the hydrochloric acid solution. 6.3072 g >>molarity = moles of solute/volume of solution (in litres) 0.45 m = n/0.4 l n = 0.45 m × 0.4 l = 0.18 mol you need 0.18 mol of. The balanced chemical equation for the partial dissociation of the base looks like this boh_text ( (aq]) rightleftharpoons b_text ( (aq])^ (+) +.
Download and Print O Come, O Come, Emmanuel sheet music for EZ Play
The balanced chemical equation for the partial dissociation of the base looks like this boh_text ( (aq]) rightleftharpoons b_text ( (aq])^ (+) +. We want the standard enthalpy of formation for ca (oh)_2. Thus, our required equation is the equation where all the constituent elements combine to. 6.3072 g >>molarity = moles of solute/volume of solution (in litres) 0.45 m.
O Come O Come Emmanuel Sheet Music Direct
6.3072 g >>molarity = moles of solute/volume of solution (in litres) 0.45 m = n/0.4 l n = 0.45 m × 0.4 l = 0.18 mol you need 0.18 mol of. The added water to reach 100.00 ml doesn't change the mols of hcl present, but it does decrease the concentration by a factor of 100//40 = 2.5. The balanced.
Free Choir Sheet Music O Come, O Come Emmanuel Michael Kravchuk
Oh− (aq) + h3o+ (aq) → 2h2o(l) so you can say that when you mix these two solutions, the hydronium cations present in the hydrochloric acid solution. The balanced chemical equation for the partial dissociation of the base looks like this boh_text ( (aq]) rightleftharpoons b_text ( (aq])^ (+) +. We want the standard enthalpy of formation for ca (oh)_2..
O Come, O Come, Emmanuel (piano) Marshall McDonald
6.3072 g >>molarity = moles of solute/volume of solution (in litres) 0.45 m = n/0.4 l n = 0.45 m × 0.4 l = 0.18 mol you need 0.18 mol of. The balanced chemical equation for the partial dissociation of the base looks like this boh_text ( (aq]) rightleftharpoons b_text ( (aq])^ (+) +. Oh− (aq) + h3o+ (aq) →.
FREE O Come O Come Emmanuel Piano Sheet Music
The balanced chemical equation for the partial dissociation of the base looks like this boh_text ( (aq]) rightleftharpoons b_text ( (aq])^ (+) +. Thus, our required equation is the equation where all the constituent elements combine to. The added water to reach 100.00 ml doesn't change the mols of hcl present, but it does decrease the concentration by a factor.
Oh Come Emmanuel Piano Letter Notes And Tin Whistle Tab Irish folk songs
Oh− (aq) + h3o+ (aq) → 2h2o(l) so you can say that when you mix these two solutions, the hydronium cations present in the hydrochloric acid solution. 6.3072 g >>molarity = moles of solute/volume of solution (in litres) 0.45 m = n/0.4 l n = 0.45 m × 0.4 l = 0.18 mol you need 0.18 mol of. The balanced.
o come emmanuel piano sheet music Come emmanuel piano music sheet cello
The balanced chemical equation for the partial dissociation of the base looks like this boh_text ( (aq]) rightleftharpoons b_text ( (aq])^ (+) +. Thus, our required equation is the equation where all the constituent elements combine to. 6.3072 g >>molarity = moles of solute/volume of solution (in litres) 0.45 m = n/0.4 l n = 0.45 m × 0.4 l.
O Come, O Come, Emmanuel Sheet Music Direct
Thus, our required equation is the equation where all the constituent elements combine to. The balanced chemical equation for the partial dissociation of the base looks like this boh_text ( (aq]) rightleftharpoons b_text ( (aq])^ (+) +. Oh− (aq) + h3o+ (aq) → 2h2o(l) so you can say that when you mix these two solutions, the hydronium cations present in.
O Come O Come Emmanuel, Chords & Lyrics
6.3072 g >>molarity = moles of solute/volume of solution (in litres) 0.45 m = n/0.4 l n = 0.45 m × 0.4 l = 0.18 mol you need 0.18 mol of. The added water to reach 100.00 ml doesn't change the mols of hcl present, but it does decrease the concentration by a factor of 100//40 = 2.5. We want.
O Come O Come Emmanuel Sheet Music PDF (Kim WalkerSmith) PraiseCharts
6.3072 g >>molarity = moles of solute/volume of solution (in litres) 0.45 m = n/0.4 l n = 0.45 m × 0.4 l = 0.18 mol you need 0.18 mol of. We want the standard enthalpy of formation for ca (oh)_2. The added water to reach 100.00 ml doesn't change the mols of hcl present, but it does decrease the.
Thus, Our Required Equation Is The Equation Where All The Constituent Elements Combine To.
6.3072 g >>molarity = moles of solute/volume of solution (in litres) 0.45 m = n/0.4 l n = 0.45 m × 0.4 l = 0.18 mol you need 0.18 mol of. We want the standard enthalpy of formation for ca (oh)_2. The added water to reach 100.00 ml doesn't change the mols of hcl present, but it does decrease the concentration by a factor of 100//40 = 2.5. The balanced chemical equation for the partial dissociation of the base looks like this boh_text ( (aq]) rightleftharpoons b_text ( (aq])^ (+) +.