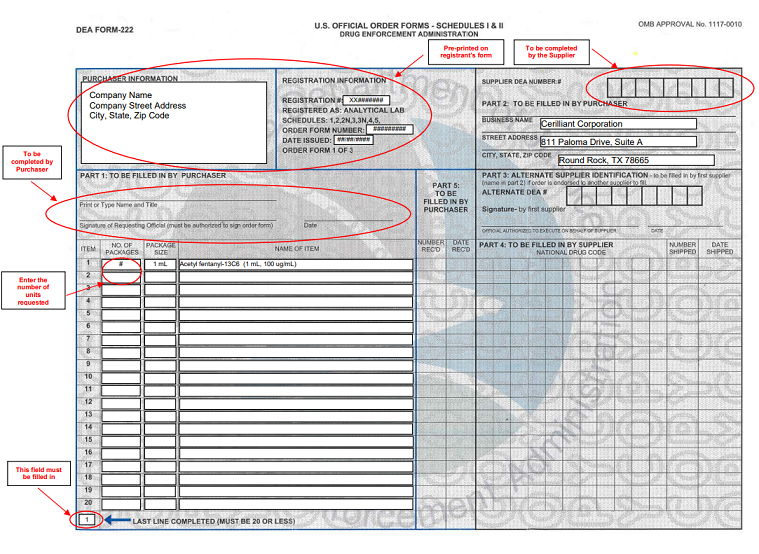
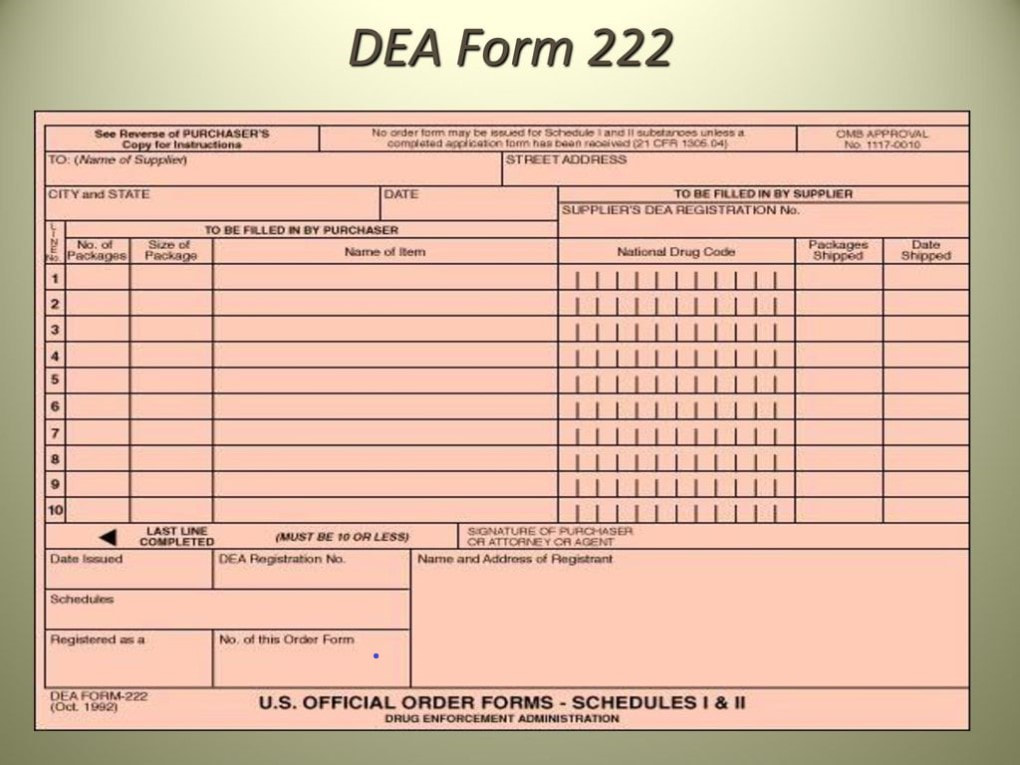
How To Fill Out Dea Form 222 - Instructions for filling out the new dea 222 form for pharmacy returns to the wholesaler of cii c2 controlled drug medications. § 1305.13 procedure for filling dea forms 222. Under 21 cfr 1305.03, the completion of dea form 222 is required for each distribution of a schedule i or ii controlled substance. 222 form orders that cannot be filled due to product availability will be held up to 60 days from form date so order can be filled when product is. Number of items and date received: (a) a purchaser must make a copy of the original dea form 222 for its records and then submit the. Once the order is received, complete the number of items and date they were received on your photocopy.
Once the order is received, complete the number of items and date they were received on your photocopy. 222 form orders that cannot be filled due to product availability will be held up to 60 days from form date so order can be filled when product is. Number of items and date received: (a) a purchaser must make a copy of the original dea form 222 for its records and then submit the. Instructions for filling out the new dea 222 form for pharmacy returns to the wholesaler of cii c2 controlled drug medications. § 1305.13 procedure for filling dea forms 222. Under 21 cfr 1305.03, the completion of dea form 222 is required for each distribution of a schedule i or ii controlled substance.
Instructions for filling out the new dea 222 form for pharmacy returns to the wholesaler of cii c2 controlled drug medications. Under 21 cfr 1305.03, the completion of dea form 222 is required for each distribution of a schedule i or ii controlled substance. Number of items and date received: § 1305.13 procedure for filling dea forms 222. Once the order is received, complete the number of items and date they were received on your photocopy. 222 form orders that cannot be filled due to product availability will be held up to 60 days from form date so order can be filled when product is. (a) a purchaser must make a copy of the original dea form 222 for its records and then submit the.
Single Sheet Dea 222 Form Instructions at Bobby Mosca blog
Under 21 cfr 1305.03, the completion of dea form 222 is required for each distribution of a schedule i or ii controlled substance. 222 form orders that cannot be filled due to product availability will be held up to 60 days from form date so order can be filled when product is. § 1305.13 procedure for filling dea forms 222..
Fillable Online DEA 222 Form Single Sheet Instructions mmscms
Once the order is received, complete the number of items and date they were received on your photocopy. Number of items and date received: (a) a purchaser must make a copy of the original dea form 222 for its records and then submit the. Instructions for filling out the new dea 222 form for pharmacy returns to the wholesaler of.
DEA222 Form Instructions MedVet International
Under 21 cfr 1305.03, the completion of dea form 222 is required for each distribution of a schedule i or ii controlled substance. Instructions for filling out the new dea 222 form for pharmacy returns to the wholesaler of cii c2 controlled drug medications. 222 form orders that cannot be filled due to product availability will be held up to.
Cerilliant Certified Reference Materials Certified Reference Standards
(a) a purchaser must make a copy of the original dea form 222 for its records and then submit the. Once the order is received, complete the number of items and date they were received on your photocopy. Under 21 cfr 1305.03, the completion of dea form 222 is required for each distribution of a schedule i or ii controlled.
Fillable Online DEA 222 Form Instructions for Schedule I & II
Under 21 cfr 1305.03, the completion of dea form 222 is required for each distribution of a schedule i or ii controlled substance. § 1305.13 procedure for filling dea forms 222. (a) a purchaser must make a copy of the original dea form 222 for its records and then submit the. Once the order is received, complete the number of.
Fillable Online Using DEA Form 222 to Order Controlled Substances Fax
Number of items and date received: Once the order is received, complete the number of items and date they were received on your photocopy. (a) a purchaser must make a copy of the original dea form 222 for its records and then submit the. Under 21 cfr 1305.03, the completion of dea form 222 is required for each distribution of.
RECORD OF DEA 222 USE Marquette University Doc Template pdfFiller
§ 1305.13 procedure for filling dea forms 222. Number of items and date received: (a) a purchaser must make a copy of the original dea form 222 for its records and then submit the. Once the order is received, complete the number of items and date they were received on your photocopy. Instructions for filling out the new dea 222.
PPT Chapter 2 PowerPoint Presentation ID250015
Once the order is received, complete the number of items and date they were received on your photocopy. Under 21 cfr 1305.03, the completion of dea form 222 is required for each distribution of a schedule i or ii controlled substance. 222 form orders that cannot be filled due to product availability will be held up to 60 days from.
DEA form 222 A Guide to the Rules and Usage
Under 21 cfr 1305.03, the completion of dea form 222 is required for each distribution of a schedule i or ii controlled substance. Number of items and date received: (a) a purchaser must make a copy of the original dea form 222 for its records and then submit the. 222 form orders that cannot be filled due to product availability.
How to Check DEA & Utilize Form 222 YouTube
§ 1305.13 procedure for filling dea forms 222. Under 21 cfr 1305.03, the completion of dea form 222 is required for each distribution of a schedule i or ii controlled substance. (a) a purchaser must make a copy of the original dea form 222 for its records and then submit the. Number of items and date received: 222 form orders.
Instructions For Filling Out The New Dea 222 Form For Pharmacy Returns To The Wholesaler Of Cii C2 Controlled Drug Medications.
Under 21 cfr 1305.03, the completion of dea form 222 is required for each distribution of a schedule i or ii controlled substance. 222 form orders that cannot be filled due to product availability will be held up to 60 days from form date so order can be filled when product is. (a) a purchaser must make a copy of the original dea form 222 for its records and then submit the. Number of items and date received:
§ 1305.13 Procedure For Filling Dea Forms 222.
Once the order is received, complete the number of items and date they were received on your photocopy.