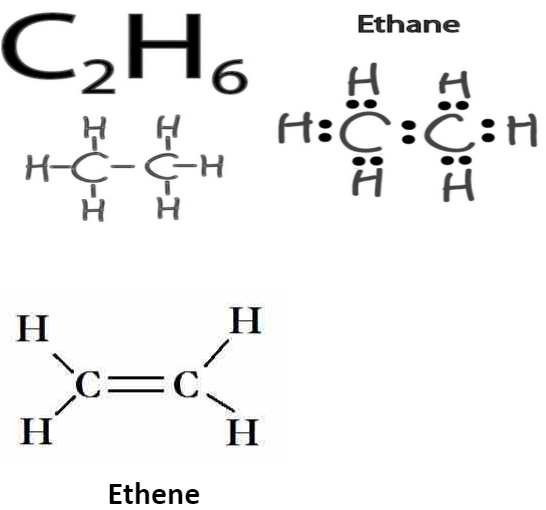
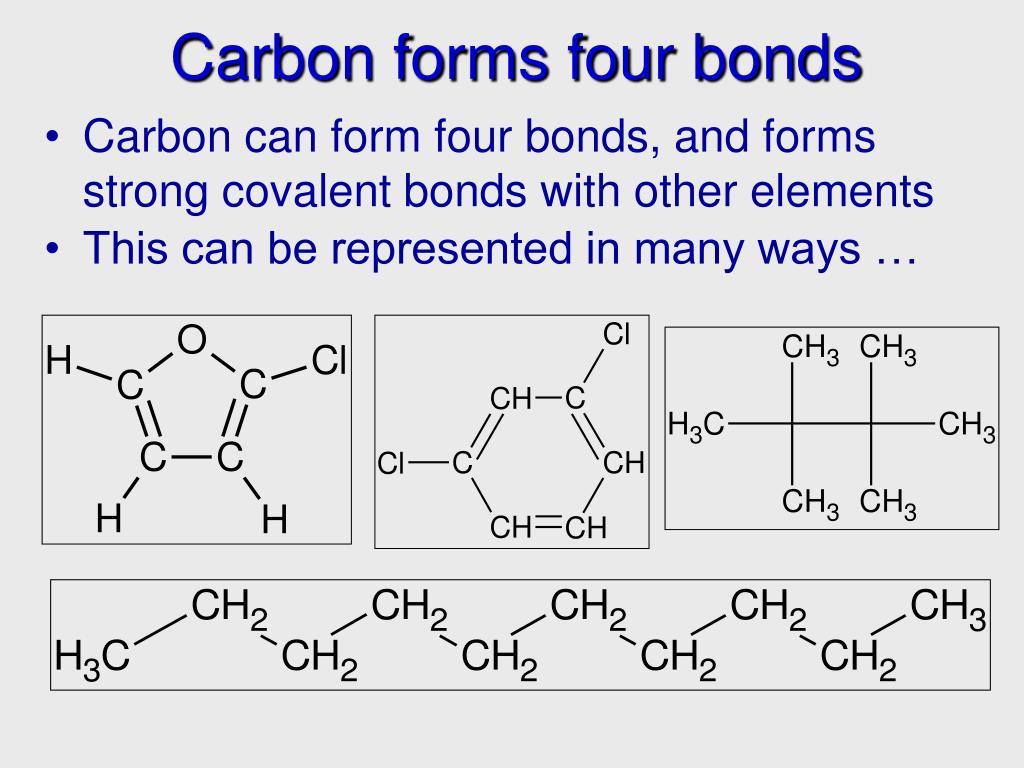
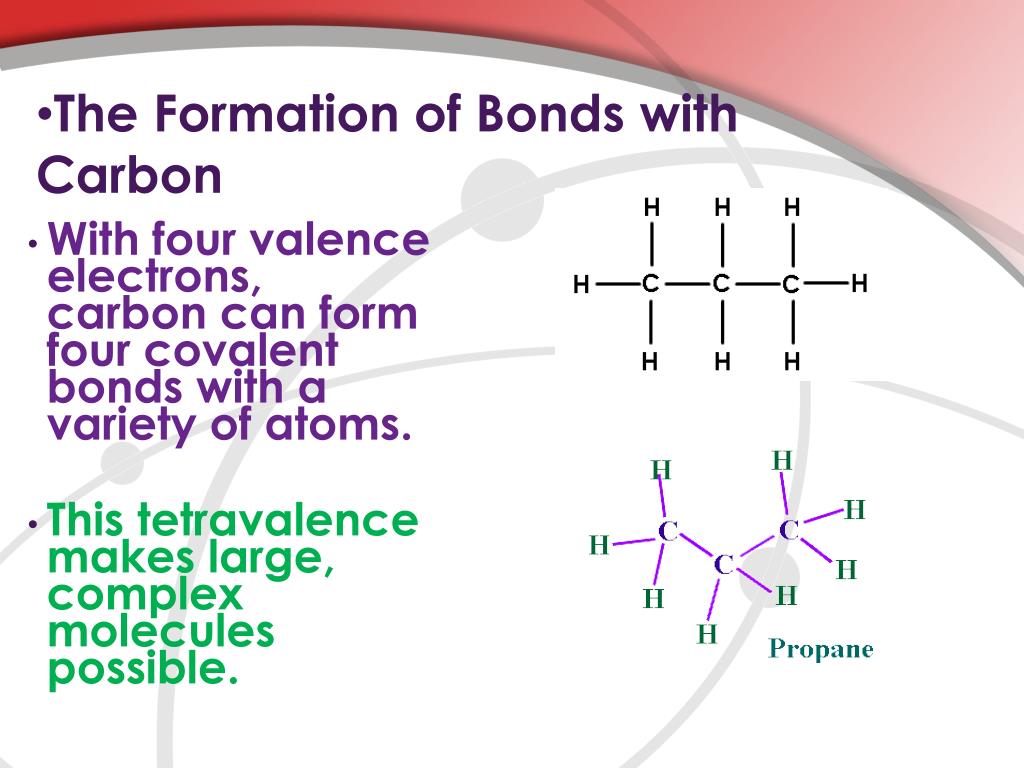
How Many Bonds Do Carbon Form - The simplest carbon molecule is methane (ch 4), depicted here. The correct answer and explanation is: Carbon can form four covalent bonds to create an organic molecule. Because carbon has four electrons in its valence (outer) shell, it can form four covalent bonds with other atoms or molecules. This allows carbon to fill its outer. How many bonds does carbon atom form. A carbon atom can form four bonds. Carbon can form up to four covalent bonds and thus share four pairs of electrons with other atoms.
Carbon can form four covalent bonds to create an organic molecule. The simplest carbon molecule is methane (ch 4), depicted here. A carbon atom can form four bonds. Because carbon has four electrons in its valence (outer) shell, it can form four covalent bonds with other atoms or molecules. Carbon can form up to four covalent bonds and thus share four pairs of electrons with other atoms. How many bonds does carbon atom form. This allows carbon to fill its outer. The correct answer and explanation is:
The correct answer and explanation is: Because carbon has four electrons in its valence (outer) shell, it can form four covalent bonds with other atoms or molecules. How many bonds does carbon atom form. The simplest carbon molecule is methane (ch 4), depicted here. A carbon atom can form four bonds. Carbon can form four covalent bonds to create an organic molecule. Carbon can form up to four covalent bonds and thus share four pairs of electrons with other atoms. This allows carbon to fill its outer.
Carbon Chemistry Carbon atoms can form single, double or triple bonds
This allows carbon to fill its outer. Carbon can form up to four covalent bonds and thus share four pairs of electrons with other atoms. The correct answer and explanation is: The simplest carbon molecule is methane (ch 4), depicted here. A carbon atom can form four bonds.
[Class 10 Chemistry] Bonding in Carbon Atoms Covalent Bonds
How many bonds does carbon atom form. A carbon atom can form four bonds. The simplest carbon molecule is methane (ch 4), depicted here. Carbon can form four covalent bonds to create an organic molecule. The correct answer and explanation is:
How To Determine The Number Of Bonds In A Molecule at Brock blog
The simplest carbon molecule is methane (ch 4), depicted here. Carbon can form four covalent bonds to create an organic molecule. The correct answer and explanation is: Because carbon has four electrons in its valence (outer) shell, it can form four covalent bonds with other atoms or molecules. How many bonds does carbon atom form.
MACROMOLECULES a.k.a. BioMolecules a.k.a. Organic Molecules ppt download
Because carbon has four electrons in its valence (outer) shell, it can form four covalent bonds with other atoms or molecules. Carbon can form four covalent bonds to create an organic molecule. This allows carbon to fill its outer. How many bonds does carbon atom form. A carbon atom can form four bonds.
Carbon Dioxide Covalent Bond
The simplest carbon molecule is methane (ch 4), depicted here. The correct answer and explanation is: Because carbon has four electrons in its valence (outer) shell, it can form four covalent bonds with other atoms or molecules. This allows carbon to fill its outer. A carbon atom can form four bonds.
How Many Forms Does Carbon Have at Claire Mcvicars blog
A carbon atom can form four bonds. Carbon can form up to four covalent bonds and thus share four pairs of electrons with other atoms. This allows carbon to fill its outer. The correct answer and explanation is: The simplest carbon molecule is methane (ch 4), depicted here.
What Covalent Bonds Can Carbon Form at Douglas Jacobson blog
This allows carbon to fill its outer. A carbon atom can form four bonds. The correct answer and explanation is: Because carbon has four electrons in its valence (outer) shell, it can form four covalent bonds with other atoms or molecules. Carbon can form four covalent bonds to create an organic molecule.
PPT Organic Chemistry Functional Groups PowerPoint Presentation
The simplest carbon molecule is methane (ch 4), depicted here. Carbon can form four covalent bonds to create an organic molecule. Carbon can form up to four covalent bonds and thus share four pairs of electrons with other atoms. Because carbon has four electrons in its valence (outer) shell, it can form four covalent bonds with other atoms or molecules..
PPT Carbon PowerPoint Presentation, free download ID1833140
Carbon can form four covalent bonds to create an organic molecule. The simplest carbon molecule is methane (ch 4), depicted here. Because carbon has four electrons in its valence (outer) shell, it can form four covalent bonds with other atoms or molecules. This allows carbon to fill its outer. A carbon atom can form four bonds.
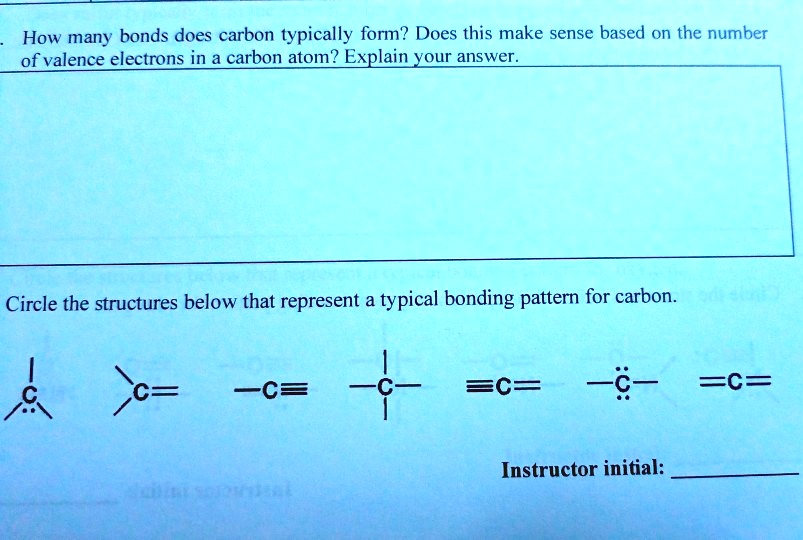
SOLVED How many bonds does carbon typically form? Does this make sense
Because carbon has four electrons in its valence (outer) shell, it can form four covalent bonds with other atoms or molecules. Carbon can form up to four covalent bonds and thus share four pairs of electrons with other atoms. This allows carbon to fill its outer. Carbon can form four covalent bonds to create an organic molecule. The simplest carbon.
A Carbon Atom Can Form Four Bonds.
Carbon can form up to four covalent bonds and thus share four pairs of electrons with other atoms. The simplest carbon molecule is methane (ch 4), depicted here. How many bonds does carbon atom form. This allows carbon to fill its outer.
The Correct Answer And Explanation Is:
Because carbon has four electrons in its valence (outer) shell, it can form four covalent bonds with other atoms or molecules. Carbon can form four covalent bonds to create an organic molecule.

![[Class 10 Chemistry] Bonding in Carbon Atoms Covalent Bonds](https://d1avenlh0i1xmr.cloudfront.net/medium/693c5ecd-56f0-42a1-aba9-2e5a5be31300/covalent-bonding-in-co2---teachoo.jpg)