Fda Form 1572 - (select one of the following.) for phase 1 investigations, a general outline of the planned. The sponsor must obtain a completed and signed 1572 before permitting an. This page provides links to commonly used clinical trial forms relevant to clinical trials. The statement of investigator, form fda 1572, is an agreement signed by the principal investigator (pi) to provide certain information to the sponsor and. When must the form be completed and signed by the investigator? For other fda forms, visit the fda forms page. Get a comprehensive understanding of fda form 1572, its purpose, and how to complete it correctly for clinical trial compliance.
For other fda forms, visit the fda forms page. When must the form be completed and signed by the investigator? This page provides links to commonly used clinical trial forms relevant to clinical trials. The sponsor must obtain a completed and signed 1572 before permitting an. The statement of investigator, form fda 1572, is an agreement signed by the principal investigator (pi) to provide certain information to the sponsor and. Get a comprehensive understanding of fda form 1572, its purpose, and how to complete it correctly for clinical trial compliance. (select one of the following.) for phase 1 investigations, a general outline of the planned.
This page provides links to commonly used clinical trial forms relevant to clinical trials. (select one of the following.) for phase 1 investigations, a general outline of the planned. The statement of investigator, form fda 1572, is an agreement signed by the principal investigator (pi) to provide certain information to the sponsor and. Get a comprehensive understanding of fda form 1572, its purpose, and how to complete it correctly for clinical trial compliance. When must the form be completed and signed by the investigator? The sponsor must obtain a completed and signed 1572 before permitting an. For other fda forms, visit the fda forms page.
PPT Regulatory Documentation Best Practices for Essential Clinical
Get a comprehensive understanding of fda form 1572, its purpose, and how to complete it correctly for clinical trial compliance. The sponsor must obtain a completed and signed 1572 before permitting an. For other fda forms, visit the fda forms page. When must the form be completed and signed by the investigator? This page provides links to commonly used clinical.
PPT Orientation for New Clinical Research PERSONNEL Module 2
When must the form be completed and signed by the investigator? For other fda forms, visit the fda forms page. The sponsor must obtain a completed and signed 1572 before permitting an. This page provides links to commonly used clinical trial forms relevant to clinical trials. The statement of investigator, form fda 1572, is an agreement signed by the principal.
Top Fda Form 1572 Templates free to download in PDF format
The sponsor must obtain a completed and signed 1572 before permitting an. (select one of the following.) for phase 1 investigations, a general outline of the planned. For other fda forms, visit the fda forms page. When must the form be completed and signed by the investigator? Get a comprehensive understanding of fda form 1572, its purpose, and how to.
Fillable Online FDA Form 1572 What It Means & Who It Includes Fax
For other fda forms, visit the fda forms page. (select one of the following.) for phase 1 investigations, a general outline of the planned. When must the form be completed and signed by the investigator? The statement of investigator, form fda 1572, is an agreement signed by the principal investigator (pi) to provide certain information to the sponsor and. This.
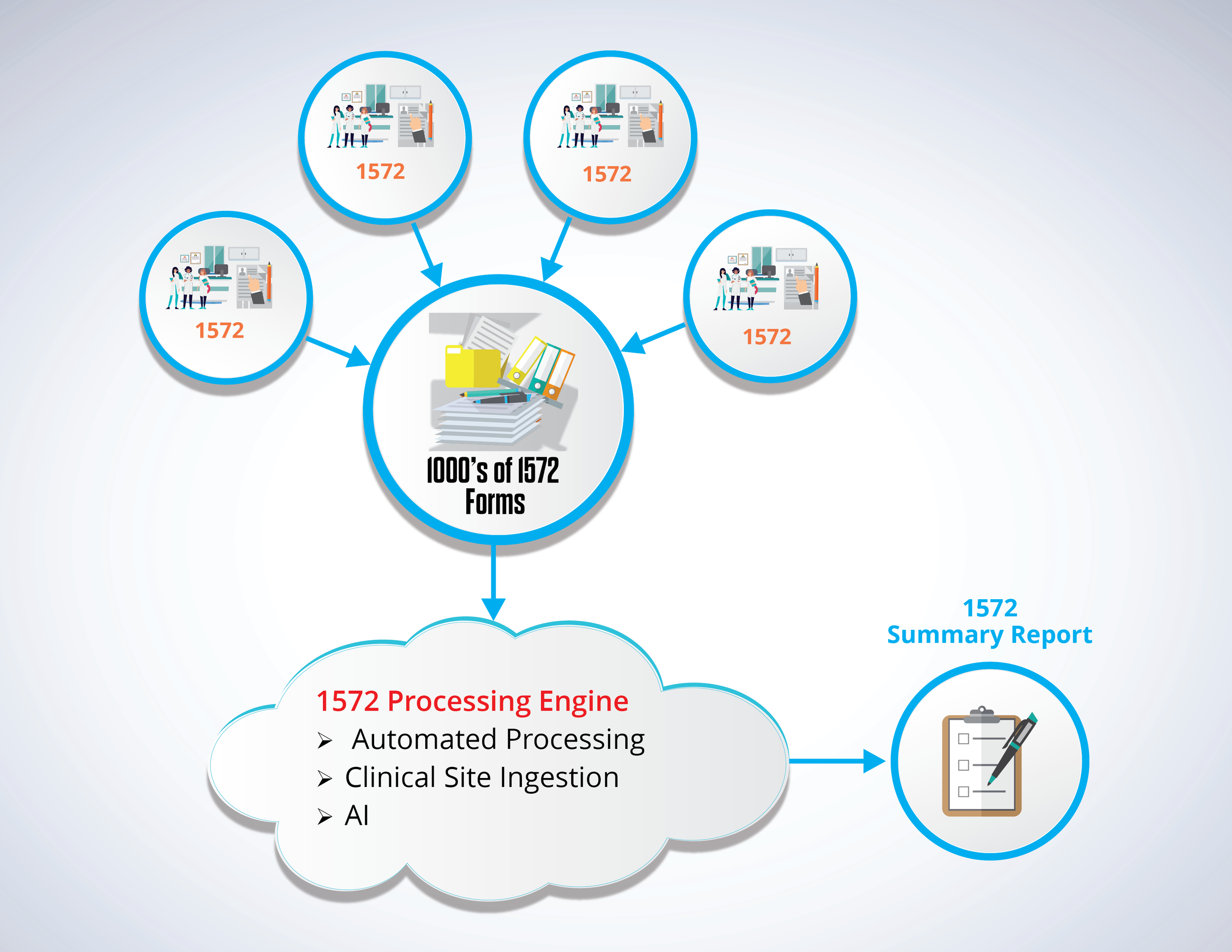
Streamlined Solution for FDA Form 1572 Summary Report Processing
For other fda forms, visit the fda forms page. (select one of the following.) for phase 1 investigations, a general outline of the planned. When must the form be completed and signed by the investigator? Get a comprehensive understanding of fda form 1572, its purpose, and how to complete it correctly for clinical trial compliance. This page provides links to.
The Ultimate Guide to Form FDA 1572 CCRPS Clinical Research
When must the form be completed and signed by the investigator? The statement of investigator, form fda 1572, is an agreement signed by the principal investigator (pi) to provide certain information to the sponsor and. This page provides links to commonly used clinical trial forms relevant to clinical trials. Get a comprehensive understanding of fda form 1572, its purpose, and.
Fda 1572 Template
This page provides links to commonly used clinical trial forms relevant to clinical trials. The statement of investigator, form fda 1572, is an agreement signed by the principal investigator (pi) to provide certain information to the sponsor and. When must the form be completed and signed by the investigator? (select one of the following.) for phase 1 investigations, a general.
PPT IND Basics in OCTGT PowerPoint Presentation, free download ID
The sponsor must obtain a completed and signed 1572 before permitting an. The statement of investigator, form fda 1572, is an agreement signed by the principal investigator (pi) to provide certain information to the sponsor and. (select one of the following.) for phase 1 investigations, a general outline of the planned. When must the form be completed and signed by.
PPT Clinical Investigator Responsibilities Regulations and
(select one of the following.) for phase 1 investigations, a general outline of the planned. The statement of investigator, form fda 1572, is an agreement signed by the principal investigator (pi) to provide certain information to the sponsor and. When must the form be completed and signed by the investigator? For other fda forms, visit the fda forms page. Get.
Fillable Fda 1572 Form Special Condition Consideration Form printable
Get a comprehensive understanding of fda form 1572, its purpose, and how to complete it correctly for clinical trial compliance. (select one of the following.) for phase 1 investigations, a general outline of the planned. The sponsor must obtain a completed and signed 1572 before permitting an. For other fda forms, visit the fda forms page. The statement of investigator,.
This Page Provides Links To Commonly Used Clinical Trial Forms Relevant To Clinical Trials.
The statement of investigator, form fda 1572, is an agreement signed by the principal investigator (pi) to provide certain information to the sponsor and. Get a comprehensive understanding of fda form 1572, its purpose, and how to complete it correctly for clinical trial compliance. The sponsor must obtain a completed and signed 1572 before permitting an. When must the form be completed and signed by the investigator?
For Other Fda Forms, Visit The Fda Forms Page.
(select one of the following.) for phase 1 investigations, a general outline of the planned.