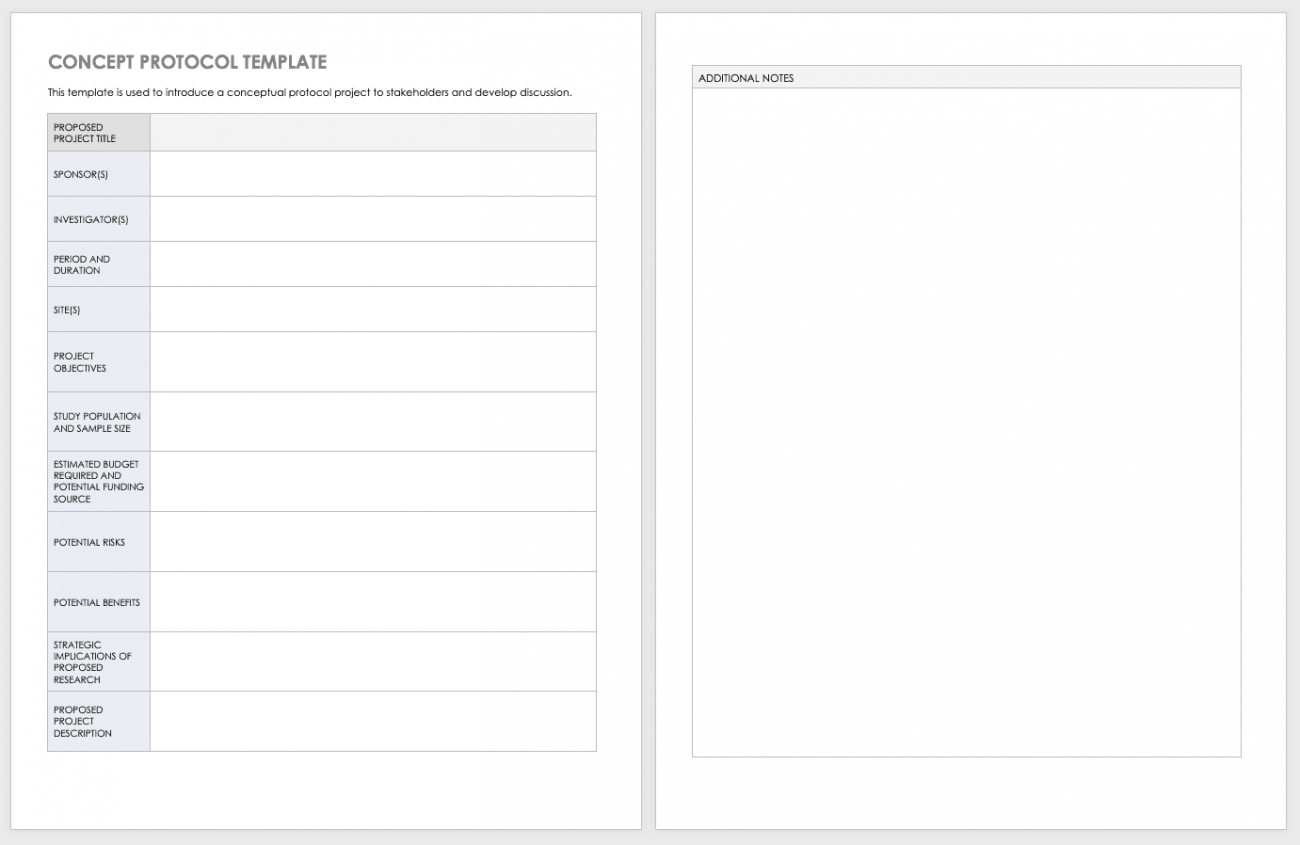
Clinical Research Protocol Template - Protocol templates for clinical trials nih applicants can use a template with instructional and sample text to help write clinical protocols for the. The irb toolkit’s registry and repository protocol template is the most efficient way for you to provide the information the irb needs. Please note that this page has been updated for 2015 following a quality check. Expedited and full board) human subjects research on this page. Clinical trial protocol template this protocol template is designed to help research teams develop a clinical trial protocol that includes an. Welcome to global health trials' tools and templates library. The purpose of this new harmonised guideline is to introduce the clinical protocol template and the technical specification to ensure. This protocol template is a tool to facilitate the development of a research study protocol specifically designed for the investigator initiated.
The purpose of this new harmonised guideline is to introduce the clinical protocol template and the technical specification to ensure. Protocol templates for clinical trials nih applicants can use a template with instructional and sample text to help write clinical protocols for the. This protocol template is a tool to facilitate the development of a research study protocol specifically designed for the investigator initiated. Expedited and full board) human subjects research on this page. Clinical trial protocol template this protocol template is designed to help research teams develop a clinical trial protocol that includes an. Please note that this page has been updated for 2015 following a quality check. Welcome to global health trials' tools and templates library. The irb toolkit’s registry and repository protocol template is the most efficient way for you to provide the information the irb needs.
Clinical trial protocol template this protocol template is designed to help research teams develop a clinical trial protocol that includes an. This protocol template is a tool to facilitate the development of a research study protocol specifically designed for the investigator initiated. The irb toolkit’s registry and repository protocol template is the most efficient way for you to provide the information the irb needs. Welcome to global health trials' tools and templates library. Protocol templates for clinical trials nih applicants can use a template with instructional and sample text to help write clinical protocols for the. Please note that this page has been updated for 2015 following a quality check. Expedited and full board) human subjects research on this page. The purpose of this new harmonised guideline is to introduce the clinical protocol template and the technical specification to ensure.
Phase 1 Clinical Trial Protocol Template
This protocol template is a tool to facilitate the development of a research study protocol specifically designed for the investigator initiated. Please note that this page has been updated for 2015 following a quality check. The irb toolkit’s registry and repository protocol template is the most efficient way for you to provide the information the irb needs. Clinical trial protocol.
Free Clinical Trial Templates Smartsheet
The irb toolkit’s registry and repository protocol template is the most efficient way for you to provide the information the irb needs. Welcome to global health trials' tools and templates library. The purpose of this new harmonised guideline is to introduce the clinical protocol template and the technical specification to ensure. Protocol templates for clinical trials nih applicants can use.
Clinical Protocol template for Investigator initiated trials (IIT
Clinical trial protocol template this protocol template is designed to help research teams develop a clinical trial protocol that includes an. The irb toolkit’s registry and repository protocol template is the most efficient way for you to provide the information the irb needs. Expedited and full board) human subjects research on this page. Protocol templates for clinical trials nih applicants.
Free Clinical Trial Templates Smartsheet
The purpose of this new harmonised guideline is to introduce the clinical protocol template and the technical specification to ensure. Please note that this page has been updated for 2015 following a quality check. Protocol templates for clinical trials nih applicants can use a template with instructional and sample text to help write clinical protocols for the. The irb toolkit’s.
Research Protocol Template Clinical Study Design
Please note that this page has been updated for 2015 following a quality check. Welcome to global health trials' tools and templates library. Protocol templates for clinical trials nih applicants can use a template with instructional and sample text to help write clinical protocols for the. The irb toolkit’s registry and repository protocol template is the most efficient way for.
Guide to writing a Clinical Trials Protocol University Hospital
Protocol templates for clinical trials nih applicants can use a template with instructional and sample text to help write clinical protocols for the. Please note that this page has been updated for 2015 following a quality check. Clinical trial protocol template this protocol template is designed to help research teams develop a clinical trial protocol that includes an. Welcome to.
Free Clinical Trial Templates Smartsheet
Please note that this page has been updated for 2015 following a quality check. This protocol template is a tool to facilitate the development of a research study protocol specifically designed for the investigator initiated. The irb toolkit’s registry and repository protocol template is the most efficient way for you to provide the information the irb needs. Welcome to global.
Phase 1 Clinical Trial Protocol Template
Expedited and full board) human subjects research on this page. The irb toolkit’s registry and repository protocol template is the most efficient way for you to provide the information the irb needs. The purpose of this new harmonised guideline is to introduce the clinical protocol template and the technical specification to ensure. Welcome to global health trials' tools and templates.
instructions for clinical research protocol template Doc Template
The purpose of this new harmonised guideline is to introduce the clinical protocol template and the technical specification to ensure. This protocol template is a tool to facilitate the development of a research study protocol specifically designed for the investigator initiated. Protocol templates for clinical trials nih applicants can use a template with instructional and sample text to help write.
Clinical Study Protocol Template
Protocol templates for clinical trials nih applicants can use a template with instructional and sample text to help write clinical protocols for the. This protocol template is a tool to facilitate the development of a research study protocol specifically designed for the investigator initiated. The irb toolkit’s registry and repository protocol template is the most efficient way for you to.
Welcome To Global Health Trials' Tools And Templates Library.
Clinical trial protocol template this protocol template is designed to help research teams develop a clinical trial protocol that includes an. This protocol template is a tool to facilitate the development of a research study protocol specifically designed for the investigator initiated. The irb toolkit’s registry and repository protocol template is the most efficient way for you to provide the information the irb needs. Please note that this page has been updated for 2015 following a quality check.
The Purpose Of This New Harmonised Guideline Is To Introduce The Clinical Protocol Template And The Technical Specification To Ensure.
Expedited and full board) human subjects research on this page. Protocol templates for clinical trials nih applicants can use a template with instructional and sample text to help write clinical protocols for the.