Can Two Metals Form An Ionic Compound - One element will lose its electron and the other. At some point you get a lot of possible phases and some means to explain why they form and not something else depending on size,. Two elements will form an ionic compound when a metal and a nonmetal will bond. Ionic bonds form when a nonmetal and a metal exchange electrons, while covalent bonds form. Do 2 metals make an ionic compound?
At some point you get a lot of possible phases and some means to explain why they form and not something else depending on size,. Ionic bonds form when a nonmetal and a metal exchange electrons, while covalent bonds form. Do 2 metals make an ionic compound? Two elements will form an ionic compound when a metal and a nonmetal will bond. One element will lose its electron and the other.
At some point you get a lot of possible phases and some means to explain why they form and not something else depending on size,. One element will lose its electron and the other. Two elements will form an ionic compound when a metal and a nonmetal will bond. Do 2 metals make an ionic compound? Ionic bonds form when a nonmetal and a metal exchange electrons, while covalent bonds form.
Lewis Dot Structures And… Ionic & Metallic Bonding. ppt download
One element will lose its electron and the other. Two elements will form an ionic compound when a metal and a nonmetal will bond. Do 2 metals make an ionic compound? Ionic bonds form when a nonmetal and a metal exchange electrons, while covalent bonds form. At some point you get a lot of possible phases and some means to.
ionic bond Definition, Properties, Examples, & Facts Britannica
Do 2 metals make an ionic compound? At some point you get a lot of possible phases and some means to explain why they form and not something else depending on size,. One element will lose its electron and the other. Ionic bonds form when a nonmetal and a metal exchange electrons, while covalent bonds form. Two elements will form.
What Pairs of Elements Form Ionic Compounds
One element will lose its electron and the other. At some point you get a lot of possible phases and some means to explain why they form and not something else depending on size,. Two elements will form an ionic compound when a metal and a nonmetal will bond. Ionic bonds form when a nonmetal and a metal exchange electrons,.
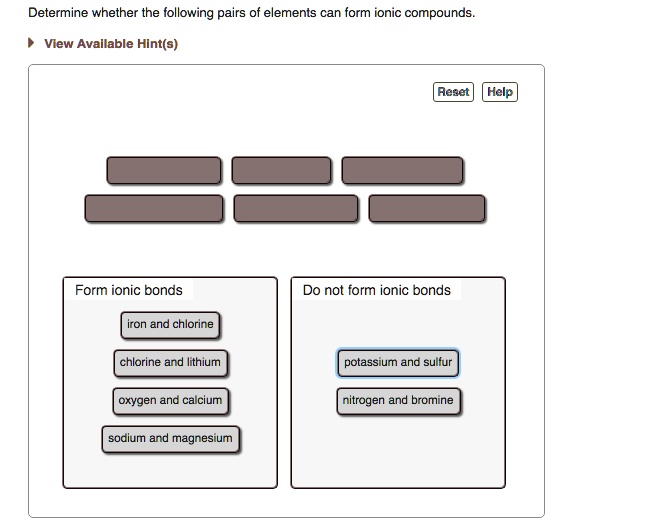
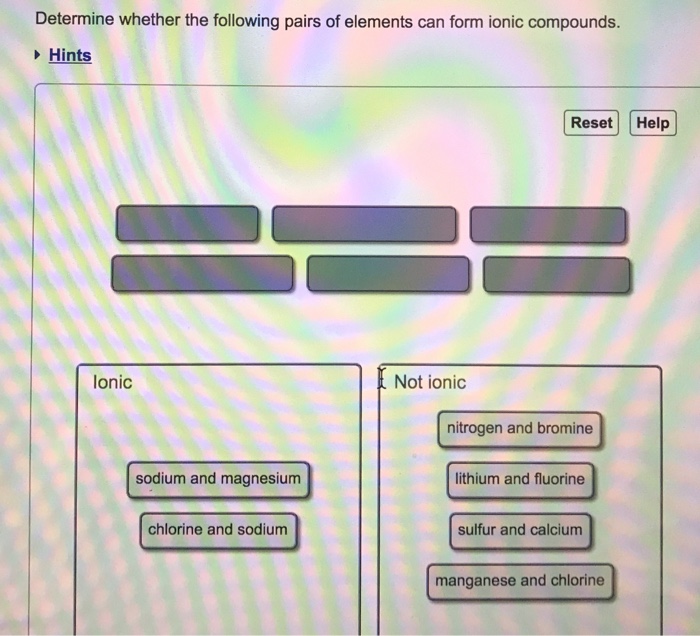
Solved Determine Whether The Following Pairs Of Elements
Two elements will form an ionic compound when a metal and a nonmetal will bond. One element will lose its electron and the other. Do 2 metals make an ionic compound? Ionic bonds form when a nonmetal and a metal exchange electrons, while covalent bonds form. At some point you get a lot of possible phases and some means to.
Chemistry Ionic Compounds. ppt download
Do 2 metals make an ionic compound? Two elements will form an ionic compound when a metal and a nonmetal will bond. Ionic bonds form when a nonmetal and a metal exchange electrons, while covalent bonds form. One element will lose its electron and the other. At some point you get a lot of possible phases and some means to.
What Is Ionic Compound Elements at Annie Geil blog
One element will lose its electron and the other. Ionic bonds form when a nonmetal and a metal exchange electrons, while covalent bonds form. Two elements will form an ionic compound when a metal and a nonmetal will bond. Do 2 metals make an ionic compound? At some point you get a lot of possible phases and some means to.
CH150 Chapter 4 Covalent Bonds and Molecular Compounds Chemistry
Do 2 metals make an ionic compound? At some point you get a lot of possible phases and some means to explain why they form and not something else depending on size,. One element will lose its electron and the other. Ionic bonds form when a nonmetal and a metal exchange electrons, while covalent bonds form. Two elements will form.
How To Form Ionic Bonds
Two elements will form an ionic compound when a metal and a nonmetal will bond. Do 2 metals make an ionic compound? Ionic bonds form when a nonmetal and a metal exchange electrons, while covalent bonds form. One element will lose its electron and the other. At some point you get a lot of possible phases and some means to.
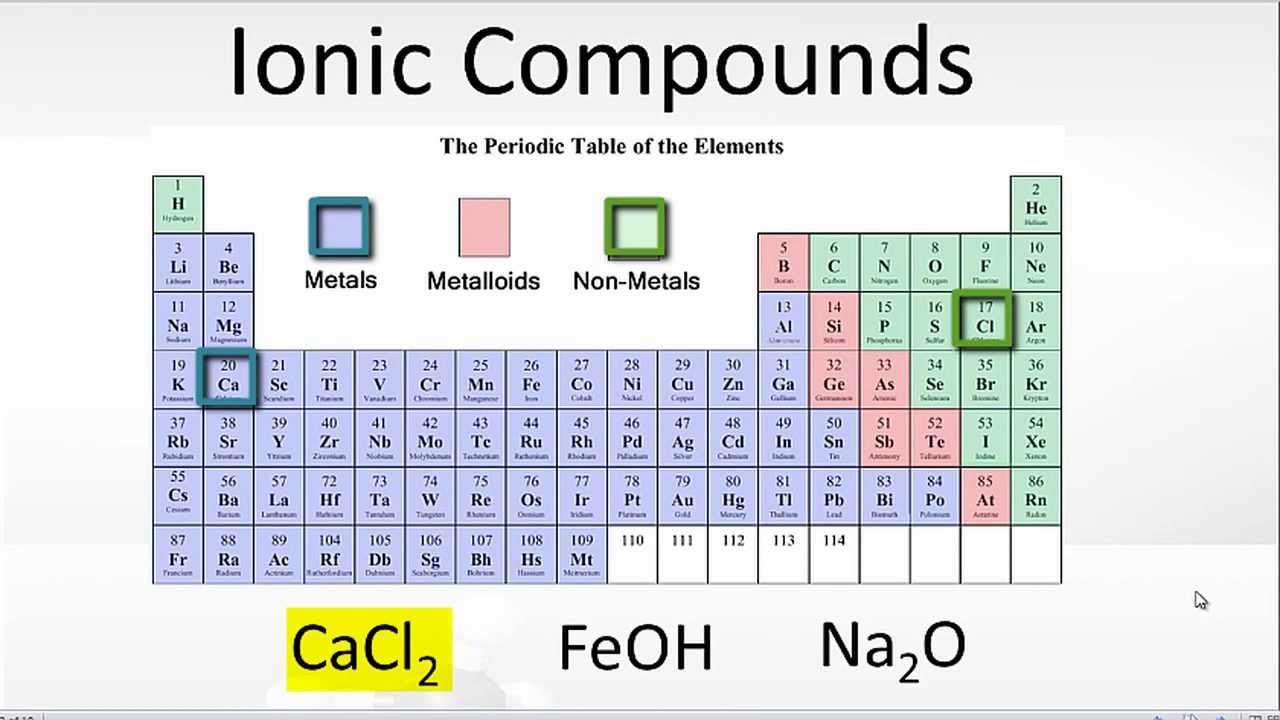
IONIC COMPOUNDS Chapter 5.8. IONIC COMPOUNDS Recall Metals form
Ionic bonds form when a nonmetal and a metal exchange electrons, while covalent bonds form. At some point you get a lot of possible phases and some means to explain why they form and not something else depending on size,. One element will lose its electron and the other. Two elements will form an ionic compound when a metal and.
Understanding Types of Chemical Bonds TEAS NurseHub
Do 2 metals make an ionic compound? Ionic bonds form when a nonmetal and a metal exchange electrons, while covalent bonds form. Two elements will form an ionic compound when a metal and a nonmetal will bond. One element will lose its electron and the other. At some point you get a lot of possible phases and some means to.
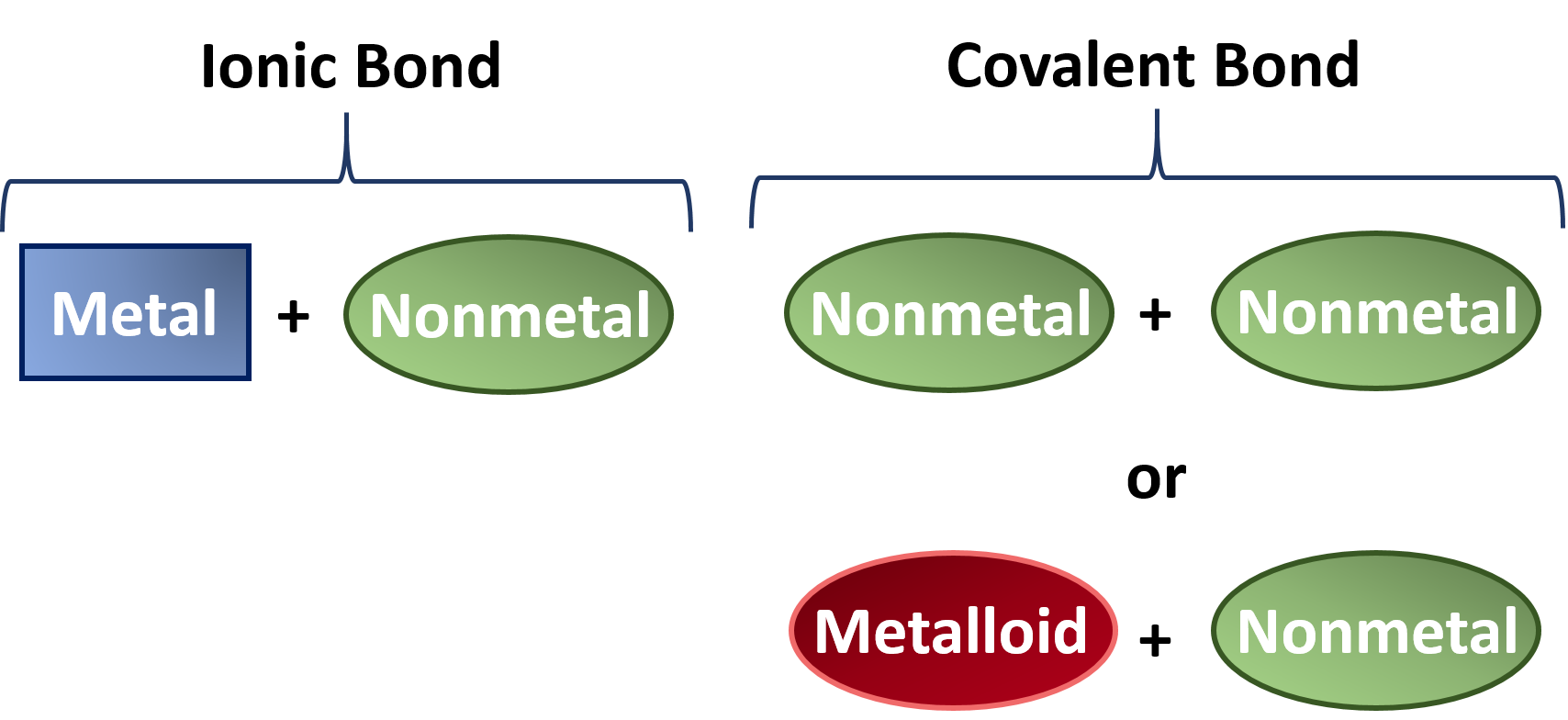
Ionic Bonds Form When A Nonmetal And A Metal Exchange Electrons, While Covalent Bonds Form.
Two elements will form an ionic compound when a metal and a nonmetal will bond. One element will lose its electron and the other. At some point you get a lot of possible phases and some means to explain why they form and not something else depending on size,. Do 2 metals make an ionic compound?